

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM10044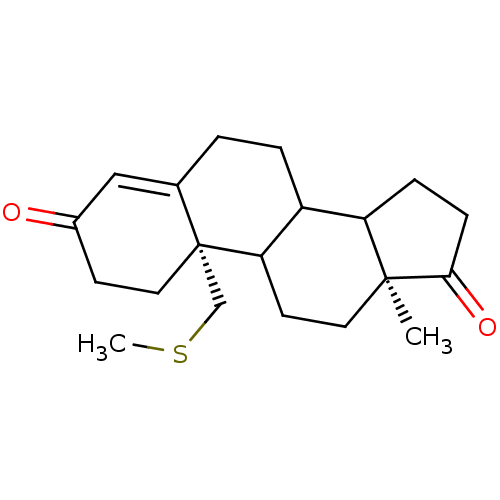 ((2S,15S)-15-methyl-2-[(methylsulfanyl)methyl]tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Aromatase inhibitor potency as iron-binding-related type II difference spectrum | J Med Chem 34: 725-36 (1991) BindingDB Entry DOI: 10.7270/Q2SB46BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10044 ((2S,15S)-15-methyl-2-[(methylsulfanyl)methyl]tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University School of Medicine Curated by ChEMBL | Assay Description Binding affinity was measured on Cytochrome P450 19A1 | J Med Chem 33: 2933-42 (1990) BindingDB Entry DOI: 10.7270/Q2VM4CW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10044 ((2S,15S)-15-methyl-2-[(methylsulfanyl)methyl]tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Centre de Recherche de Roussel Uclaf | Assay Description The enzyme activity was assayed by measuring the formation of tritiated water from [1beta, 2beta-3H ]androstenedione in the presence of increasing co... | J Med Chem 39: 757-72 (1996) Article DOI: 10.1021/jm950539l BindingDB Entry DOI: 10.7270/Q28C9TGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||