

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50002134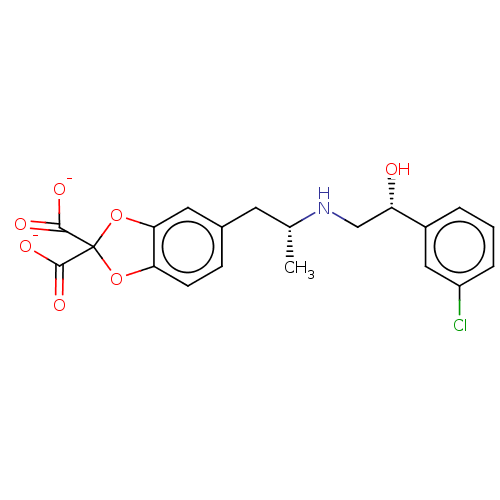 (5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Rattus norvegicus) | BDBM50002134 (5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Agonistic activity towards beta-2 adrenoceptor. Mean concentration required to produce 50% inhibition of uterine contraction | J Med Chem 44: 1436-45 (2001) BindingDB Entry DOI: 10.7270/Q2Q52QVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Rattus norvegicus) | BDBM50002134 (5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Beta-2 adrenergic receptor in rat soleus membrane by displacing (-)-isoproterenol (50 microM) | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50002134 (5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.62E+5 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Agonistic activity was assessed by measurement of cAMP accumulation levels in CHO cells expressing human beta-2-adrenergic receptor | Bioorg Med Chem Lett 11: 757-60 (2001) BindingDB Entry DOI: 10.7270/Q2BP022B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||