 Found 7 hits Enz. Inhib. hit(s) with Target = 'Beta-2 adrenergic receptor' and Ligand = 'BDBM50131281'
Found 7 hits Enz. Inhib. hit(s) with Target = 'Beta-2 adrenergic receptor' and Ligand = 'BDBM50131281' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50131281
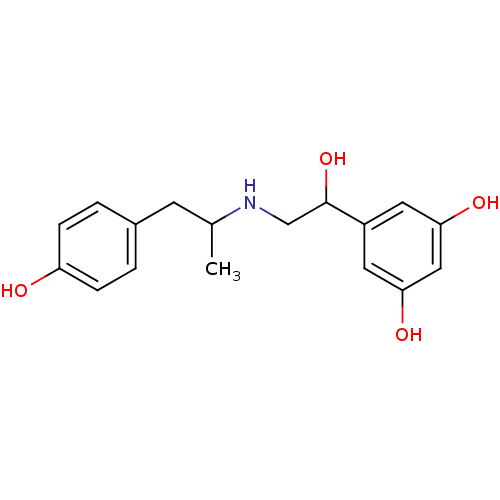
(5-(1-hydroxy-2-(1-(4-hydroxyphenyl)propan-2-ylamin...)Show InChI InChI=1S/C17H21NO4/c1-11(6-12-2-4-14(19)5-3-12)18-10-17(22)13-7-15(20)9-16(21)8-13/h2-5,7-9,11,17-22H,6,10H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50131281

(5-(1-hydroxy-2-(1-(4-hydroxyphenyl)propan-2-ylamin...)Show InChI InChI=1S/C17H21NO4/c1-11(6-12-2-4-14(19)5-3-12)18-10-17(22)13-7-15(20)9-16(21)8-13/h2-5,7-9,11,17-22H,6,10H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 665-74 (1994)
BindingDB Entry DOI: 10.7270/Q2RN36CJ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50131281

(5-(1-hydroxy-2-(1-(4-hydroxyphenyl)propan-2-ylamin...)Show InChI InChI=1S/C17H21NO4/c1-11(6-12-2-4-14(19)5-3-12)18-10-17(22)13-7-15(20)9-16(21)8-13/h2-5,7-9,11,17-22H,6,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 719 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 369: 151-9 (2004)
Article DOI: 10.1007/s00210-003-0860-y
BindingDB Entry DOI: 10.7270/Q2PZ57C6 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50131281

(5-(1-hydroxy-2-(1-(4-hydroxyphenyl)propan-2-ylamin...)Show InChI InChI=1S/C17H21NO4/c1-11(6-12-2-4-14(19)5-3-12)18-10-17(22)13-7-15(20)9-16(21)8-13/h2-5,7-9,11,17-22H,6,10H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mainz
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound Beta-2 adrenergic receptor in Guinea pig trachea |
Bioorg Med Chem Lett 13: 2687-92 (2003)
BindingDB Entry DOI: 10.7270/Q22N51P2 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50131281

(5-(1-hydroxy-2-(1-(4-hydroxyphenyl)propan-2-ylamin...)Show InChI InChI=1S/C17H21NO4/c1-11(6-12-2-4-14(19)5-3-12)18-10-17(22)13-7-15(20)9-16(21)8-13/h2-5,7-9,11,17-22H,6,10H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino
Curated by ChEMBL
| Assay Description
Agonist activity at adrenergic beta-2 receptor in guinea pig tracheal rings assessed as myorelaxing activity on carbachol-induced contraction in pres... |
J Med Chem 50: 5003-11 (2007)
Article DOI: 10.1021/jm0704595
BindingDB Entry DOI: 10.7270/Q28P61B5 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50131281

(5-(1-hydroxy-2-(1-(4-hydroxyphenyl)propan-2-ylamin...)Show InChI InChI=1S/C17H21NO4/c1-11(6-12-2-4-14(19)5-3-12)18-10-17(22)13-7-15(20)9-16(21)8-13/h2-5,7-9,11,17-22H,6,10H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino
Curated by ChEMBL
| Assay Description
Agonist activity at adrenergic beta-2 receptor in guinea pig tracheal rings assessed as myorelaxing activity on carbachol-induced contraction |
J Med Chem 50: 5003-11 (2007)
Article DOI: 10.1021/jm0704595
BindingDB Entry DOI: 10.7270/Q28P61B5 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50131281

(5-(1-hydroxy-2-(1-(4-hydroxyphenyl)propan-2-ylamin...)Show InChI InChI=1S/C17H21NO4/c1-11(6-12-2-4-14(19)5-3-12)18-10-17(22)13-7-15(20)9-16(21)8-13/h2-5,7-9,11,17-22H,6,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina
Curated by ChEMBL
| Assay Description
Binding affinity to beta-2 adrenergic receptor (unknown origin) at 1 to 10000 nM |
Bioorg Med Chem Lett 23: 5376-81 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.052
BindingDB Entry DOI: 10.7270/Q2F76GHV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data