

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50002134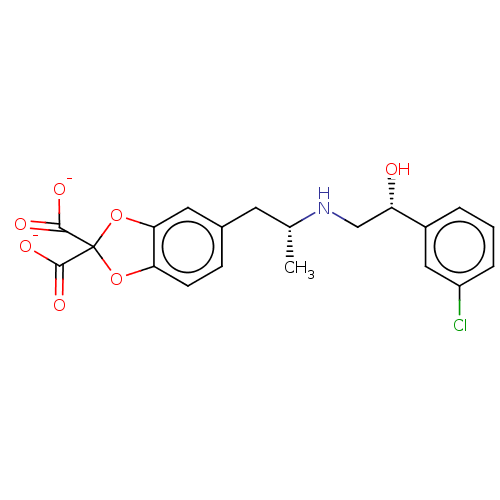 (5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to CHO cells expressing the cloned human Beta-3 adrenergic receptor in the presence of [125I]iodocyan... | Bioorg Med Chem Lett 11: 3035-9 (2001) BindingDB Entry DOI: 10.7270/Q2MS3T97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50002134 (5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Agonistic activity as cAMP accumulation in CHO cells expressing human beta-3-andrenergic receptor | Bioorg Med Chem Lett 11: 757-60 (2001) BindingDB Entry DOI: 10.7270/Q2BP022B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50002134 (5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Agonist activity at Homo sapiens (human) beta3 adrenoreceptor | Med Chem Res 19: 1121-1140 (2010) Article DOI: 10.1007/s00044-009-9257-x BindingDB Entry DOI: 10.7270/Q2057JTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Rattus norvegicus) | BDBM50002134 (5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company Ltd. Curated by ChEMBL | Assay Description Agonistic activity towards beta-3 adrenoceptor. Mean concentration required to produce 50% relaxation of detrusor before the addition in the ferret d... | J Med Chem 44: 1436-45 (2001) BindingDB Entry DOI: 10.7270/Q2Q52QVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Rattus norvegicus) | BDBM50002134 (5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated in vitro for beta-adrenergic activity against beta-3 adrenergic receptor in rat epididymal fat pads by stimulation of glycerol... | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||