

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM210070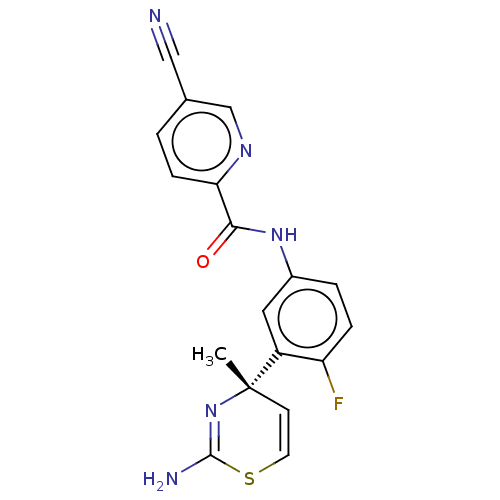 (US9270353, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] JNJ-962 from BACE1 (unknown origin) expressed in HEK293 cell membranes assessed as inhibition constant at pH 6.2 by scintillatio... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00359 BindingDB Entry DOI: 10.7270/Q2WW7NJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM210070 (US9270353, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 in human SH-SY5Y cells expressing wild type betaAPP assessed as reduction in amyloidbeta (1 to 40 residues) level incubated for 2... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01917 BindingDB Entry DOI: 10.7270/Q2445R8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Mus musculus (Mouse)) | BDBM210070 (US9270353, 17) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse BACE1 by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01917 BindingDB Entry DOI: 10.7270/Q2445R8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM210070 (US9270353, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Biological activity against Oxytocin receptor in rat | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM210070 (US9270353, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 (43 to 454 residues) expressed in Escherichia coli BL21 (DE3) using Biotin-XSEVNLDAEFRHDSGC-Eu fluorogenic pept... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01917 BindingDB Entry DOI: 10.7270/Q2445R8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM210070 (US9270353, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description 48.5 μL of substrate peptide solution (Biotin-XSEVNLDAEFRHDSGC-Eu: X=ε-amino-n-capronic acid, Eu=Europium cryptate) was added to each well ... | US Patent US9273053 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||