

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM19483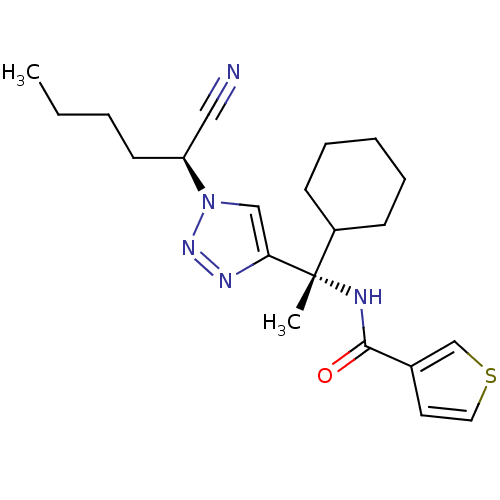 (1, 2, 3 -Triazole Nitrile Inhibitor, 11d | N-[(1S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | -46.0 | n/a | n/a | n/a | n/a | n/a | 6.1 | 37 |
University of California at Berkeley | Assay Description The proteolytic cleavage of N-acyl aminocoumarins by cathepsins was conducted in Dynatech Microfluor fluorescence 96-well microtiter plates, and read... | J Med Chem 49: 6298-307 (2006) Article DOI: 10.1021/jm060701s BindingDB Entry DOI: 10.7270/Q2GF0RSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19483 (1, 2, 3 -Triazole Nitrile Inhibitor, 11d | N-[(1S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S using FR-aminoluciferin as substrate preincubated for 15 mins before substrate addition measured after 1 hr by luminescence... | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19483 (1, 2, 3 -Triazole Nitrile Inhibitor, 11d | N-[(1S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Inhibition of cathepsin S-mediated antigen presentation in B/T hybridoma cells assessed as IL-2 level | Bioorg Med Chem Lett 22: 7189-93 (2012) Article DOI: 10.1016/j.bmcl.2012.09.054 BindingDB Entry DOI: 10.7270/Q2R212JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||