

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM367643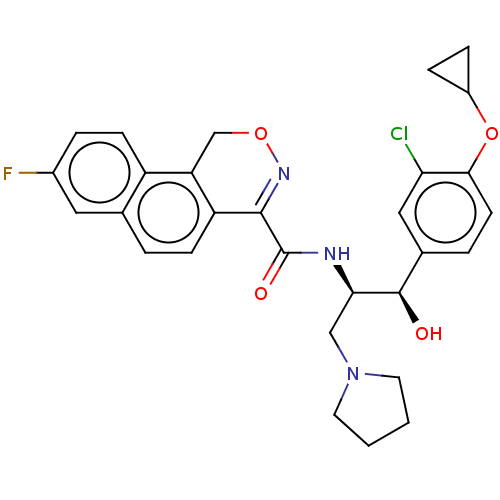 (N-((1R,2R)-1-(3-chloro-4- cyclopropoxyphenyl)-1-hy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Protein concentration was determined using BCA assay kit. Sixty micrograms of MDCK cell lysate was incubated with various concentrations of a compoun... | US Patent US10759769 (2020) BindingDB Entry DOI: 10.7270/Q2GT5R8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM367643 (N-((1R,2R)-1-(3-chloro-4- cyclopropoxyphenyl)-1-hy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description This assay was modified based on the study by Larsen et al. (J. Lipid Res. 2011, 53, 282). Madin-Darby canine kidney (MDCK) cell lysate was prepared ... | J Med Chem 51: 1319-23 (2008) BindingDB Entry DOI: 10.7270/Q2G44SK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||