

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50329178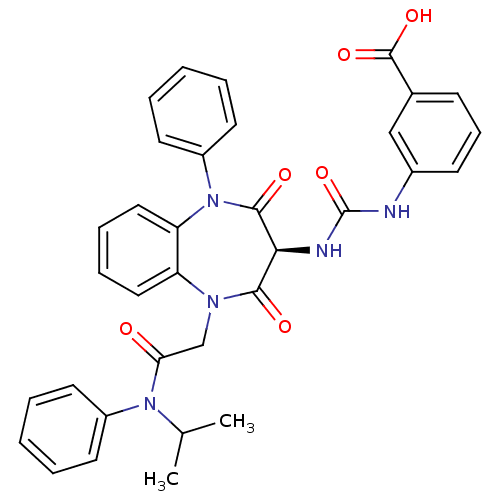 ((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CCK8 from rat CCK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6797-801 (2010) Article DOI: 10.1016/j.bmcl.2010.08.115 BindingDB Entry DOI: 10.7270/Q2TH8MZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50329178 ((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Curated by ChEMBL | Assay Description Displacement of [125I]CCK from CCK1R (unknown origin) expressed in CHO cells | Bioorg Med Chem Lett 25: 1849-55 (2015) Article DOI: 10.1016/j.bmcl.2015.03.051 BindingDB Entry DOI: 10.7270/Q2SJ1NB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50329178 ((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Compound was tested in vitro for its ability to displace [125I]Bolton-Hunter CCK-8 from membrane preparation isolated from CHO-KI cells stably transf... | J Med Chem 39: 5236-45 (1997) Article DOI: 10.1021/jm9601664 BindingDB Entry DOI: 10.7270/Q2PG1T0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50329178 ((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Curated by ChEMBL | Assay Description Displacement of [125I]BDZ1 from CCK1R (unknown origin) expressed in CHO cells | Bioorg Med Chem Lett 25: 1849-55 (2015) Article DOI: 10.1016/j.bmcl.2015.03.051 BindingDB Entry DOI: 10.7270/Q2SJ1NB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50329178 ((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CCK8 from human CCK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 20: 6797-801 (2010) Article DOI: 10.1016/j.bmcl.2010.08.115 BindingDB Entry DOI: 10.7270/Q2TH8MZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50329178 ((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 63.8 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at rat CCK1 receptor expressed in CHO cells assessed as induction of calcium release by FLIPR assay | Bioorg Med Chem Lett 20: 6797-801 (2010) Article DOI: 10.1016/j.bmcl.2010.08.115 BindingDB Entry DOI: 10.7270/Q2TH8MZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50329178 ((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 283 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at human CCK1 receptor expressed in CHO cells assessed as induction of calcium release by FLIPR assay | Bioorg Med Chem Lett 20: 6797-801 (2010) Article DOI: 10.1016/j.bmcl.2010.08.115 BindingDB Entry DOI: 10.7270/Q2TH8MZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50329178 ((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
Mayo Clinic Curated by ChEMBL | Assay Description Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 1 nM (Rvb = 7 to 14 pM) | Bioorg Med Chem Lett 25: 1849-55 (2015) Article DOI: 10.1016/j.bmcl.2015.03.051 BindingDB Entry DOI: 10.7270/Q2SJ1NB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50329178 ((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a |
Mayo Clinic Curated by ChEMBL | Assay Description Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 0.316 nM (Rvb = 7 to 14... | Bioorg Med Chem Lett 25: 1849-55 (2015) Article DOI: 10.1016/j.bmcl.2015.03.051 BindingDB Entry DOI: 10.7270/Q2SJ1NB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50329178 ((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Mayo Clinic Curated by ChEMBL | Assay Description Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 0.1 nM (Rvb = 7 to 14 p... | Bioorg Med Chem Lett 25: 1849-55 (2015) Article DOI: 10.1016/j.bmcl.2015.03.051 BindingDB Entry DOI: 10.7270/Q2SJ1NB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50329178 ((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a |
Mayo Clinic Curated by ChEMBL | Assay Description Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 0.01 nM (Rvb = 7 to 14 ... | Bioorg Med Chem Lett 25: 1849-55 (2015) Article DOI: 10.1016/j.bmcl.2015.03.051 BindingDB Entry DOI: 10.7270/Q2SJ1NB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50329178 ((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Mayo Clinic Curated by ChEMBL | Assay Description Activity at CCK1R (unknown origin) expressed in CHO cells assessed as stimulation of intracellular calcium responses | Bioorg Med Chem Lett 25: 1849-55 (2015) Article DOI: 10.1016/j.bmcl.2015.03.051 BindingDB Entry DOI: 10.7270/Q2SJ1NB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||