

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50418610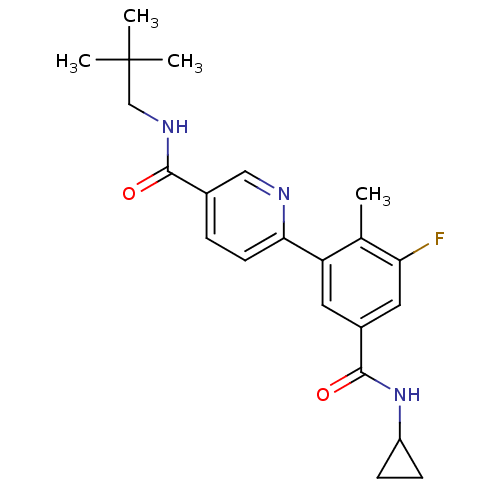 (GW-856553 | GW856553X | LOSMAPIMOD | US10550073, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co., Ltd. US Patent | Assay Description Experimental Method: 4-hydroxydiclofenac (the substrate for CYP450 and 2C9 enzymes) and different doses of compounds were added into human liver micr... | US Patent US10550073 (2020) BindingDB Entry DOI: 10.7270/Q21R6SWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50418610 (GW-856553 | GW856553X | LOSMAPIMOD | US10550073, C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 expressed in Escherichia coli by fluorimetric assay | J Med Chem 52: 6257-69 (2009) Article DOI: 10.1021/jm9004779 BindingDB Entry DOI: 10.7270/Q2J967NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||