 Found 11 hits Enz. Inhib. hit(s) with Target = 'Cytochrome P450 3A4' and Ligand = 'BDBM25817'
Found 11 hits Enz. Inhib. hit(s) with Target = 'Cytochrome P450 3A4' and Ligand = 'BDBM25817' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM25817
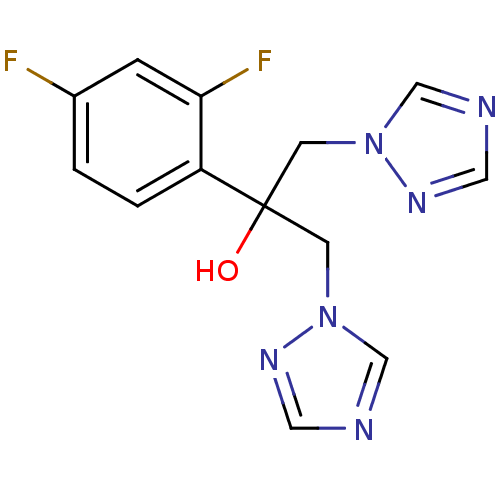
(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
US Patent
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Viamet Pharmaceuticals, Inc.
US Patent
| Assay Description
Solutions of each test compound were separately prepared at concentrations of 20000, 6000, 2000, 600, 200, and 60 μM by serial dilution with DMS... |
US Patent US8883797 (2014)
BindingDB Entry DOI: 10.7270/Q2FT8JRF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM25817

(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
US Patent
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Viamet Pharmaceuticals, Inc.
US Patent
| |
US Patent US9556143 (2017)
BindingDB Entry DOI: 10.7270/Q2NG4SMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM25817

(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
US Patent
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mycovia Pharmaceuticals, Inc.
US Patent
| Assay Description
Pooled human liver microsome suspension (20 mg/mL) was diluted with phosphate buffer to obtain a 5 mg/mL suspension. A solution of NADPH was prepared... |
US Patent US10221160 (2019)
BindingDB Entry DOI: 10.7270/Q2736T6Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM25817

(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
US Patent
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Pooled human liver microsome suspension (20 mg/mL) was diluted with phosphate buffer to obtain a 5 mg/mL suspension. A solution of NADPH was prepared... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F76GSF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM25817

(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
US Patent
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Viamet Pharmaceuticals, Inc.
US Patent
| Assay Description
Solutions of each test compound were separately prepared at
concentrations of 20000, 6000, 2000, 600, 200, and 60 uM by serial
dilution with DMSO:M... |
US Patent US9221791 (2015)
BindingDB Entry DOI: 10.7270/Q24M93B6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM25817

(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
US Patent
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter & Gamble Company
US Patent
| Assay Description
Cytochrome P450 is a large and diverse group of enzymes that catalyze the oxidation of organic substances. Some members of the CYP family contribute ... |
US Patent US9144538 (2015)
BindingDB Entry DOI: 10.7270/Q22806DV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM25817

(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
US Patent
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Procter & Gamble Company
US Patent
| Assay Description
A commercially available P450-GLO Assay kit (Promega Corporation, Madison Wis.) is used to screen various compounds for CYP3A4A inhibition activity. ... |
US Patent US9138393 (2015)
BindingDB Entry DOI: 10.7270/Q2GF0S8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM25817

(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel Aviv University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP3A4 using Luciferin-PPXE as substrate preincubated for 10 mins followed by NADPH addition measured after 15 mins b... |
Eur J Med Chem 179: 779-790 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.003
BindingDB Entry DOI: 10.7270/Q2TX3JP3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM25817

(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Eur J Med Chem 44: 2913-22 (2009)
Article DOI: 10.1016/j.ejmech.2008.12.004
BindingDB Entry DOI: 10.7270/Q2NC6174 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM25817

(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 29: 2016-2024 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.040
BindingDB Entry DOI: 10.7270/Q2N01B1G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM25817

(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Viamet Pharmaceuticals Inc., Durham, NC 27703, USA. Electronic address: cyates@viamet.com.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human hepatocyte microsomes using testosterone substrate by HPLC/MS/MS method |
Bioorg Med Chem Lett 27: 3243-3248 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.037
BindingDB Entry DOI: 10.7270/Q29889G6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data