

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50002238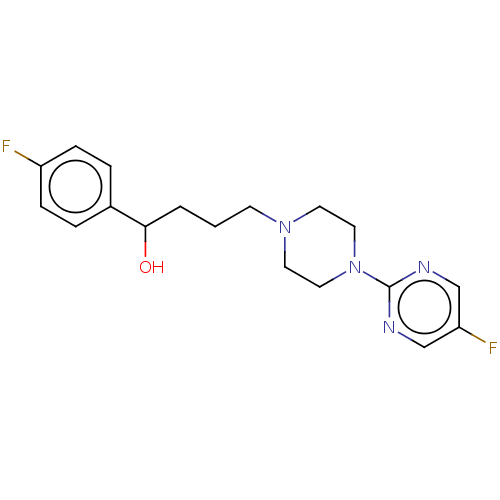 ((R)1-(4-Fluoro-phenyl)-4-[4-(5-fluoro-pyrimidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against radioligand [3H](-)-sulpiride binding to Dopamine receptor D2 in rat | J Med Chem 42: 1076-87 (1999) Article DOI: 10.1021/jm980212v BindingDB Entry DOI: 10.7270/Q29C6Z4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50002238 ((R)1-(4-Fluoro-phenyl)-4-[4-(5-fluoro-pyrimidin-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Du Pont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description In vitro binding affinity towards dopamine receptor D2 | J Med Chem 35: 4344-61 (1992) BindingDB Entry DOI: 10.7270/Q28P614G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50002238 ((R)1-(4-Fluoro-phenyl)-4-[4-(5-fluoro-pyrimidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against the Dopamine receptor D2 in rat striatum by using [3H]spiperone radioligand | Bioorg Med Chem Lett 2: 165-170 (1992) Article DOI: 10.1016/S0960-894X(01)80443-1 BindingDB Entry DOI: 10.7270/Q28P610P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50002238 ((R)1-(4-Fluoro-phenyl)-4-[4-(5-fluoro-pyrimidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description In vitro inhibitory against radioligand [3H]spiperone binding to rat striatal Dopamine receptor D2 | J Med Chem 35: 4516-25 (1993) BindingDB Entry DOI: 10.7270/Q2H70GDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50002238 ((R)1-(4-Fluoro-phenyl)-4-[4-(5-fluoro-pyrimidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 binding site using [3H]spiroperidol. | J Med Chem 38: 1998-2008 (1995) BindingDB Entry DOI: 10.7270/Q2SJ1M8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||