

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057767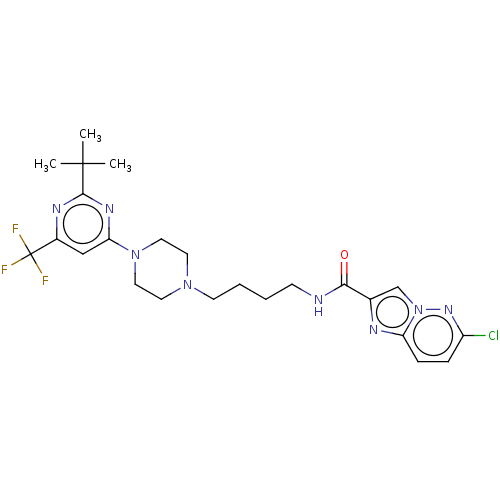 (CHEMBL3323017 | US9598387, Compound 116 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057767 (CHEMBL3323017 | US9598387, Compound 116 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Roma La Sapienza | Assay Description Stably transfected HEK cells expressing the human D2-long and the D3 dopamine receptor were developed using the pIRESneo2 bicistronic expression vect... | J Med Chem 52: 1935-42 (2009) BindingDB Entry DOI: 10.7270/Q2HD7Z0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057767 (CHEMBL3323017 | US9598387, Compound 116 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute US Patent | Assay Description The affinities of the target compounds were determined using radioligand binding experiments. All radioligand binding experiments were performed acco... | US Patent US9598387 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057767 (CHEMBL3323017 | US9598387, Compound 116 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against D3R in human U2OS cells assessed as inhibition of (+)-PD128907-induced beta-arrestin translocation by beta-galactosidase ... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057767 (CHEMBL3323017 | US9598387, Compound 116 | US996974...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 637 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity against human D3R expressed in CHO cells assessed as inhibition of quinpirole-induced mitogenesis after 24 hrs by [3H]thymidine u... | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||