

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000518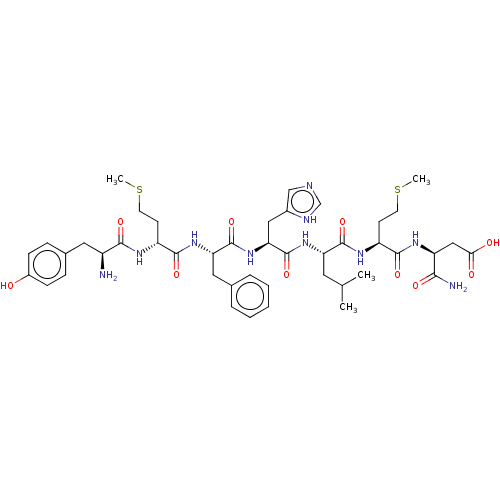 (3-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Environmental Health Sciences Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 in P2 membrane preparation of rat brain by [3H]DADLE displacement. | J Med Chem 34: 1350-5 (1991) BindingDB Entry DOI: 10.7270/Q2ZW1MHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000518 (3-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Environmental Health Sciences Curated by ChEMBL | Assay Description Compound was tested for its binding affinity against opioid receptor delta using [3H]-DPDPE as radioligand. | J Med Chem 35: 1222-7 (1992) BindingDB Entry DOI: 10.7270/Q29P30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000518 (3-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Inhibition of [3H]- ]DSLET binding to delta receptor from rat brain membrane | J Med Chem 35: 3956-61 (1992) BindingDB Entry DOI: 10.7270/Q2H70DRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000518 (3-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Inhibition of [3H]- ]DSLET binding to delta receptor from rat brain membrane | J Med Chem 35: 3956-61 (1992) BindingDB Entry DOI: 10.7270/Q2H70DRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000518 (3-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.695 | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Inhibition of [3H]- ]DSLET binding to opioid receptor delta from rat brain membrane | J Med Chem 35: 3956-61 (1992) BindingDB Entry DOI: 10.7270/Q2H70DRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000518 (3-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 98.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Inhibition of electrically evoked contractions in mouse vas deferens | J Med Chem 35: 3956-61 (1992) BindingDB Entry DOI: 10.7270/Q2H70DRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||