

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin receptor type B (Sus scrofa) | BDBM50061096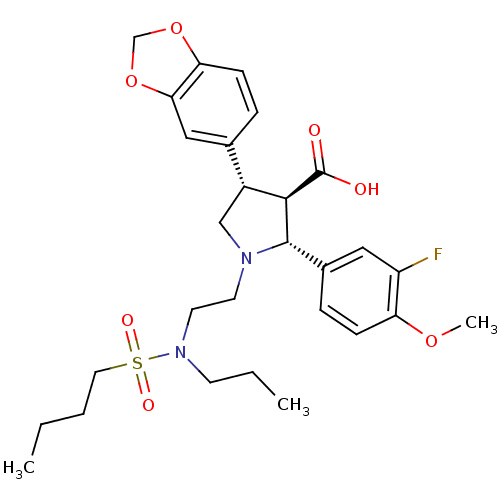 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-{2-[(butane-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Ability to displace endothelin ([125I]-ET-3) from endothelin B receptor derived from cerebellar tissue of porcine. | J Med Chem 40: 3217-27 (1997) Article DOI: 10.1021/jm970101g BindingDB Entry DOI: 10.7270/Q2C24VJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Sus scrofa) | BDBM50061096 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-{2-[(butane-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Ability to displace endothelin ([125I]-ET-3) from endothelin B receptor derived from cerebellar tissue of porcine. | J Med Chem 40: 3217-27 (1997) Article DOI: 10.1021/jm970101g BindingDB Entry DOI: 10.7270/Q2C24VJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50061096 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-{2-[(butane-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding assay performed using human Endothelin B receptor (hETB) expressed in chinese hamster ovary cells(CHO) | J Med Chem 40: 3217-27 (1997) Article DOI: 10.1021/jm970101g BindingDB Entry DOI: 10.7270/Q2C24VJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50061096 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-{2-[(butane-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Ability of the compound to block the ET-1-induced hydrolysis of inositol phosphate in Endothelin B receptor of chinese hamster ovary(CHO) cells. | J Med Chem 40: 3217-27 (1997) Article DOI: 10.1021/jm970101g BindingDB Entry DOI: 10.7270/Q2C24VJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||