

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heparanase (Homo sapiens (Human)) | BDBM50147526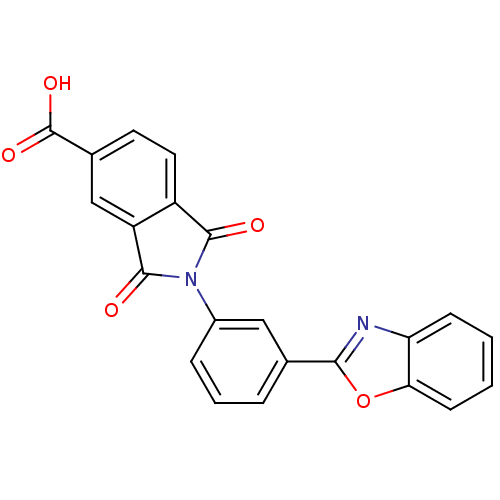 (2-(3-(benzo[d]oxazol-2-yl)phenyl)-1,3-dioxoisoindo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sharif University of Technology Curated by ChEMBL | Assay Description Inhibition of heparanase | Eur J Med Chem 43: 548-56 (2008) Article DOI: 10.1016/j.ejmech.2007.04.014 BindingDB Entry DOI: 10.7270/Q2PC33KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147526 (2-(3-(benzo[d]oxazol-2-yl)phenyl)-1,3-dioxoisoindo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es Curated by ChEMBL | Assay Description Inhibition of recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) | Bioorg Med Chem 21: 1944-51 (2013) Article DOI: 10.1016/j.bmc.2013.01.033 BindingDB Entry DOI: 10.7270/Q2HQ419G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147526 (2-(3-(benzo[d]oxazol-2-yl)phenyl)-1,3-dioxoisoindo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147526 (2-(3-(benzo[d]oxazol-2-yl)phenyl)-1,3-dioxoisoindo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147526 (2-(3-(benzo[d]oxazol-2-yl)phenyl)-1,3-dioxoisoindo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||