

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM35228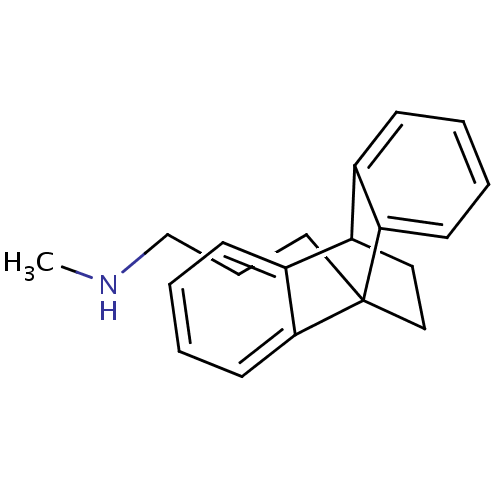 (CHEMBL21731 | Dibencycladine | Ludiomil | maprotil...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation Curated by PDSP Ki Database | Brain Res 304: 1-7 (1984) Article DOI: 10.1016/0006-8993(84)90856-4 BindingDB Entry DOI: 10.7270/Q21N7ZMS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM35228 (CHEMBL21731 | Dibencycladine | Ludiomil | maprotil...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe Universit£t Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from human histamine H1 receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 538-42 (2008) Article DOI: 10.1016/j.bmcl.2008.09.012 BindingDB Entry DOI: 10.7270/Q25H7H6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||