

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H1 receptor (RAT) | BDBM50008984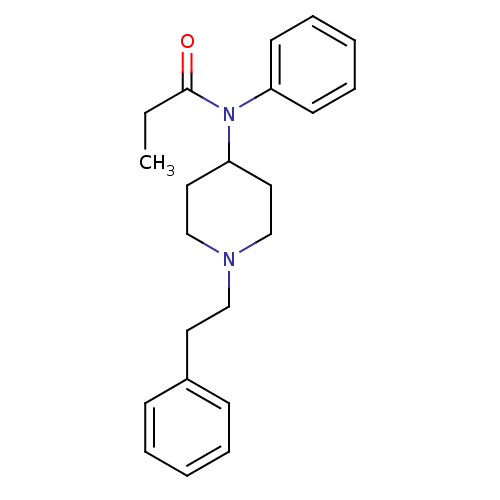 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Displacement of [3H]mepyramine from histamine H1 receptor in Sprague-Dawley rat brain membrane after 2 hr by scintillation counting | J Med Chem 55: 7054-60 (2012) Article DOI: 10.1021/jm300671m BindingDB Entry DOI: 10.7270/Q2FN17BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50008984 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Antagonist activity at H1 receptor in human HeLa cells assessed as inhibition of histamine-induced Ca2+ release by using fura-2AM-based fluorescence ... | J Med Chem 55: 7054-60 (2012) Article DOI: 10.1021/jm300671m BindingDB Entry DOI: 10.7270/Q2FN17BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||