

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50503690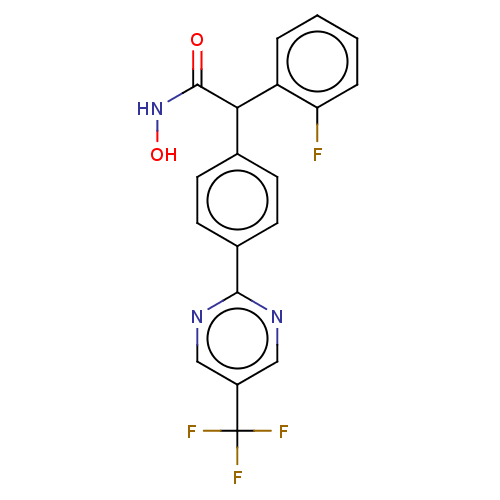 (CHEMBL4456445) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t D£sseldorf Curated by ChEMBL | Assay Description Inhibition of recombinant human HDAC4 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115108 BindingDB Entry DOI: 10.7270/Q2NV9NSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50503690 (CHEMBL4456445) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles River Discovery (previously BioFocus) Curated by ChEMBL | Assay Description Inhibition of HDAC4 catalytic domain (648 to 1057 residues) (unknown origin) using Boc-Lys-TFA as substrate measured after 60 mins by fluorescence as... | Bioorg Med Chem Lett 29: 83-88 (2019) Article DOI: 10.1016/j.bmcl.2018.11.009 BindingDB Entry DOI: 10.7270/Q2G44TJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50503690 (CHEMBL4456445) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01149 BindingDB Entry DOI: 10.7270/Q2CC14PT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50503690 (CHEMBL4456445) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal GST-fusion tagged/C-terminal GST-tagged HDAC4 (627 to 1084 residues) expressed in baculovirus infected ins... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113095 BindingDB Entry DOI: 10.7270/Q22Z1991 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50503690 (CHEMBL4456445) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a |
Charles River Discovery (previously BioFocus) Curated by ChEMBL | Assay Description Binding affinity to HDAC4 catalytic domain (648 to 1057 residues) (unknown origin) by surface plasmon resonance analysis | Bioorg Med Chem Lett 29: 83-88 (2019) Article DOI: 10.1016/j.bmcl.2018.11.009 BindingDB Entry DOI: 10.7270/Q2G44TJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||