

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM65817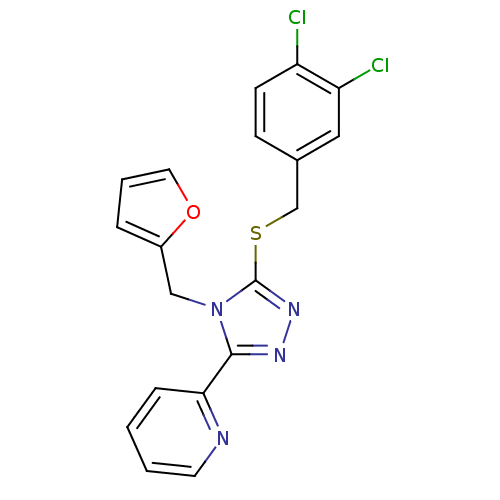 (2-[5-[(3,4-dichlorobenzyl)thio]-4-(2-furfuryl)-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Departments of Molecular Medicine and Neuroscience, The Scripps Research Institute, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assay | ACS Med Chem Lett 8: 694-700 (2017) Article DOI: 10.1021/acsmedchemlett.7b00224 BindingDB Entry DOI: 10.7270/Q23N25TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM65817 (2-[5-[(3,4-dichlorobenzyl)thio]-4-(2-furfuryl)-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics Source Affiliation: Sanford-Burnham Medical Research Institute Network: NIH Molecular Lib... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2X63KC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM65817 (2-[5-[(3,4-dichlorobenzyl)thio]-4-(2-furfuryl)-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 347 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics Source Affiliation: Sanford-Burnham Medical Research Institute Network: NIH Molecular Lib... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2HX1B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM65817 (2-[5-[(3,4-dichlorobenzyl)thio]-4-(2-furfuryl)-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 867 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2D50KDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM65817 (2-[5-[(3,4-dichlorobenzyl)thio]-4-(2-furfuryl)-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 870 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) assessed as increase in beta arrestin 2 recruitment | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111701 BindingDB Entry DOI: 10.7270/Q2668HN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM65817 (2-[5-[(3,4-dichlorobenzyl)thio]-4-(2-furfuryl)-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.99E+3 | n/a | n/a | n/a | n/a |
Departments of Molecular Medicine and Neuroscience, The Scripps Research Institute, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as beta-arrestin2 recruitment by enzyme fragment complementation meth... | ACS Med Chem Lett 8: 694-700 (2017) Article DOI: 10.1021/acsmedchemlett.7b00224 BindingDB Entry DOI: 10.7270/Q23N25TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM65817 (2-[5-[(3,4-dichlorobenzyl)thio]-4-(2-furfuryl)-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 870 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in human U2OS cells co-transfected with EFC and beta-arrestin-2 assessed as increase in bet... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM65817 (2-[5-[(3,4-dichlorobenzyl)thio]-4-(2-furfuryl)-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) assessed as increase in beta arrestin 2 recruitment relative to control | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111701 BindingDB Entry DOI: 10.7270/Q2668HN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||