

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254919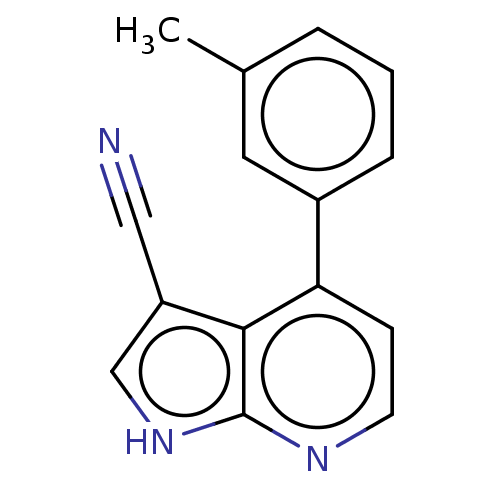 (US9499542, 2 | US9675594, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254919 (US9499542, 2 | US9675594, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 G2019S mutant (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrat... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254919 (US9499542, 2 | US9675594, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of human wild type LRRK2 phosphorylation at ser935 transfected in HEK293 cells after 48 hrs by in-cell Western assay | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254919 (US9499542, 2 | US9675594, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254919 (US9499542, 2 | US9675594, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 8.5 | 30 |
H. Lundbeck A/S; Vernalis (R&D) Ltd. US Patent | Assay Description LRRK2 kinase activity is measured using a LanthaScreen kinase activity assay available from Invitrogen (Life Technologies Corporation). The assay is ... | US Patent US9499542 (2016) BindingDB Entry DOI: 10.7270/Q22N516S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||