

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271309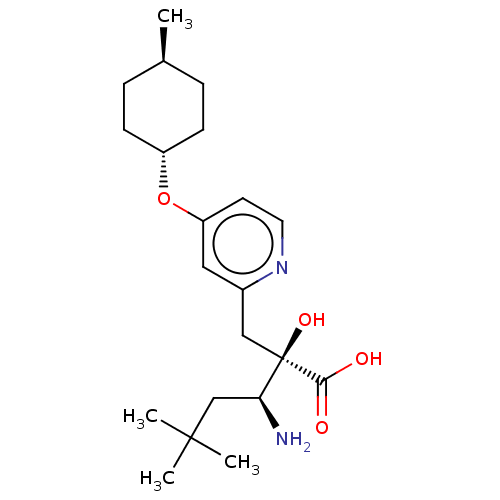 (US10059720, Example 124 | US10975091, Example 124) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271309 (US10059720, Example 124 | US10975091, Example 124) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271309 (US10059720, Example 124 | US10975091, Example 124) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description hP-LAP: HEK293 cells forced to transiently express hP-LAP were prepared by lipofection, homogenized, and then subjected to ultracentrifugation at 100... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271309 (US10059720, Example 124 | US10975091, Example 124) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||