

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM101271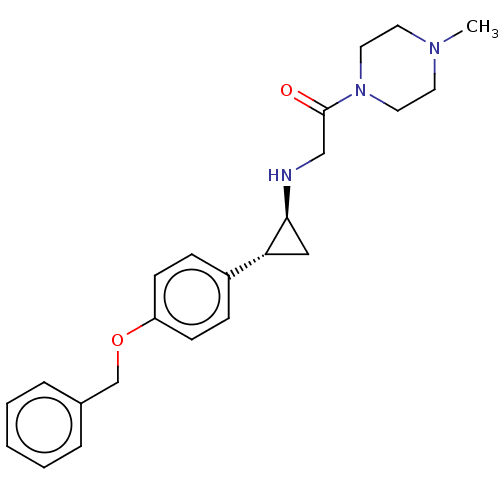 (US8524717, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oryzon Genomics, S.A. US Patent | Assay Description Biological assay using monoamine oxidase or LSD1. | US Patent US8524717 (2013) BindingDB Entry DOI: 10.7270/Q26H4G1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM101271 (US8524717, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of N-terminal truncated recombinant human LSD1 (151 to 852 residues) expressed in Escherichia coli using histone H3 (1 to 21 residues) K4m... | Bioorg Med Chem 26: 4871-4880 (2018) Article DOI: 10.1016/j.bmc.2018.08.026 BindingDB Entry DOI: 10.7270/Q2PR7ZN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM101271 (US8524717, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Freiburg Curated by ChEMBL | Assay Description Inhibition of LSD1 (unknown origin) | Eur J Med Chem 144: 52-67 (2018) Article DOI: 10.1016/j.ejmech.2017.12.001 BindingDB Entry DOI: 10.7270/Q20V8GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||