

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50233715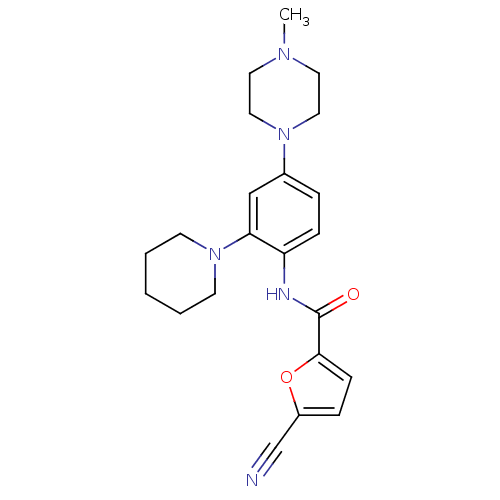 (5-cyano-N-(4-(4-methylpiperazin-1-yl)-2-(piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of FMS mediated phosphorylation using SYEGNSYTFIDPTQ as substrate after 80 mins by fluorescence polarization | J Med Chem 54: 7860-83 (2011) Article DOI: 10.1021/jm200900q BindingDB Entry DOI: 10.7270/Q2VD70JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50233715 (5-cyano-N-(4-(4-methylpiperazin-1-yl)-2-(piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01571 BindingDB Entry DOI: 10.7270/Q25H7MBX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50233715 (5-cyano-N-(4-(4-methylpiperazin-1-yl)-2-(piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic FMS expressed in Sf9-baculovirus system after 80 mins by fluorescence polarization | Bioorg Med Chem Lett 18: 1642-8 (2008) Article DOI: 10.1016/j.bmcl.2008.01.059 BindingDB Entry DOI: 10.7270/Q2FB52PS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||