

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM463444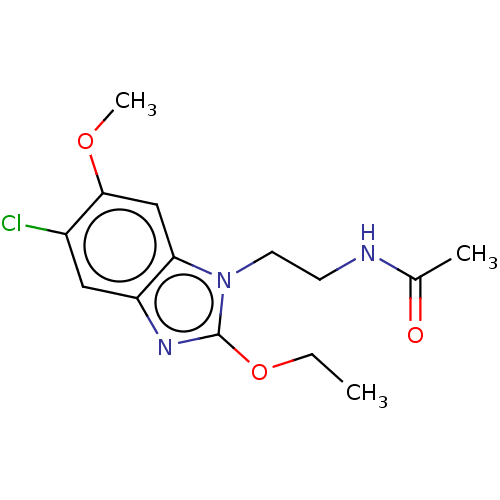 (US10781182, Compound IA2-121 | US11091445, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ACHÉ LABORATÓRIOS FARMACÊUTICOS S.A. US Patent | Assay Description The binding assay was performed in melatonergic MT1 and MT2 receptors in order to check the receptor affinity for the ligand, i.e., the ability of th... | US Patent US10781182 (2020) BindingDB Entry DOI: 10.7270/Q2JH3Q7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM463444 (US10781182, Compound IA2-121 | US11091445, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding assay was performed in melatonergic MT1 and MT2 receptors in order to check the receptor affinity for the ligand, i.e., the ability of th... | Citation and Details BindingDB Entry DOI: 10.7270/Q24Q7Z44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM463444 (US10781182, Compound IA2-121 | US11091445, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[1251]-iodomelatonin from human MT1 receptor expressed in CHO cells incubated for 60 mins by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM463444 (US10781182, Compound IA2-121 | US11091445, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human MT1 receptor expressed in CHO cells assessed as increase in forskolin induced cAMP production incubated at 37 degreeC by Hi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM463444 (US10781182, Compound IA2-121 | US11091445, Compoun...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human MT1 receptor expressed in CHO cells assessed as increase in cAMP levels incubated at 28 degreeC by cellular dielectric spec... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00627 BindingDB Entry DOI: 10.7270/Q2Z03CZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||