

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM360881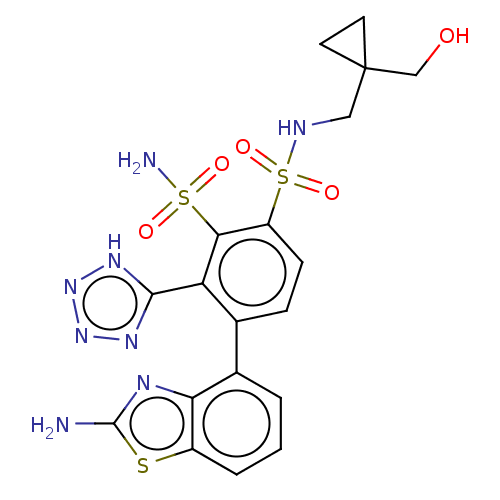 (4-(2-amino-1,3- benzothiazol-4-yl)-N1-{[1- (hydrox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Klebsiella pneumoniae) | BDBM360881 (4-(2-amino-1,3- benzothiazol-4-yl)-N1-{[1- (hydrox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM360881 (4-(2-amino-1,3- benzothiazol-4-yl)-N1-{[1- (hydrox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.287 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM360881 (4-(2-amino-1,3- benzothiazol-4-yl)-N1-{[1- (hydrox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.287 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||