

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM495191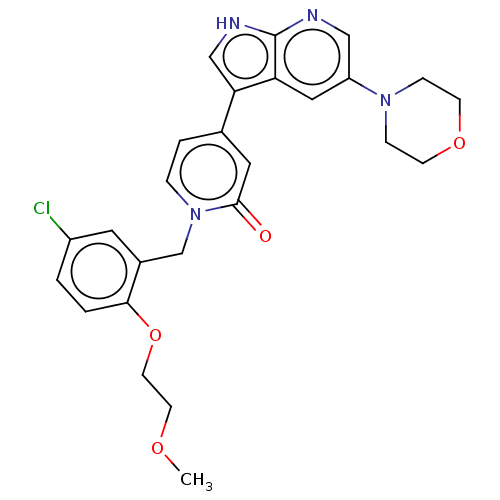 (1-(5-Chloro-2-(2-methoxyethoxy)benzyl)-4-(5-morpho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DZ0DDB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM495191 (1-(5-Chloro-2-(2-methoxyethoxy)benzyl)-4-(5-morpho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <150 | n/a | n/a | n/a | n/a | n/a | n/a |
AGV DISCOVERY; INSTITUT NATIONAL DE LA SANTE ET DE LA RECHERCHE MEDICALE (INSERM); CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE (CNRS); UNIVERSITE DE MONTPELLIER US Patent | Assay Description To assess compounds capacity to inhibit ERK2 enzymatic activity, Z′-Lyte biochemical assay from Life technologies was used according to manufac... | US Patent US10995089 (2021) BindingDB Entry DOI: 10.7270/Q2W95D9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||