

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21121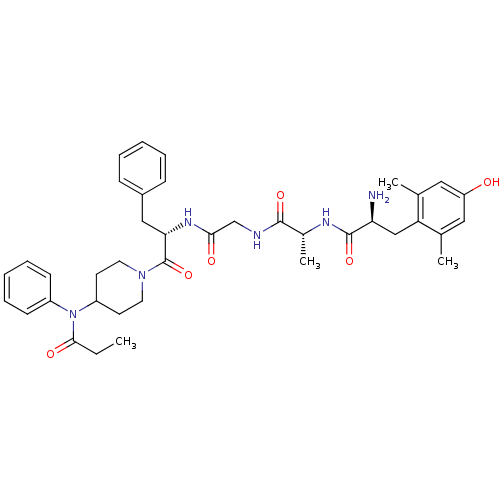 ((2S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.380 | -53.8 | n/a | n/a | 0.880 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 5528-32 (2007) Article DOI: 10.1021/jm061465o BindingDB Entry DOI: 10.7270/Q2D50K8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21121 ((2S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21121 ((2S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in mouse HN9.10 cells | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21121 ((2S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-[(...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig ileum assessed as inhibition of electrically-stimulated muscle contraction | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21121 ((2S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||