

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003367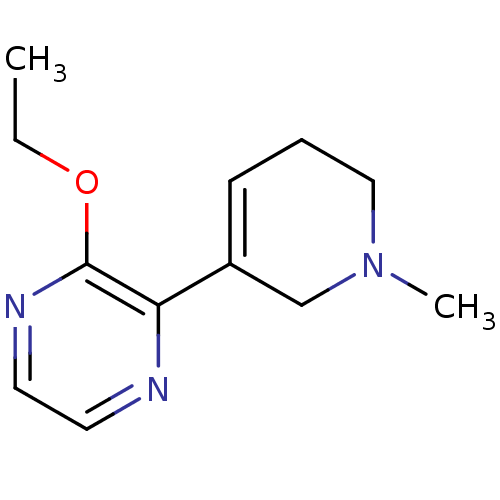 (2-Ethoxy-3-(1-methyl-1,2,5,6-tetrahydro-pyridin-3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against rat hippocampus M1 receptor using [3H]-pirenzepine (Pz) as radioligand | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50003367 (2-Ethoxy-3-(1-methyl-1,2,5,6-tetrahydro-pyridin-3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against rat hippocampus M1 receptor using [3H]-oxotremorine-M (Oxo-M) as radioligand | J Med Chem 35: 4011-9 (1992) BindingDB Entry DOI: 10.7270/Q2KW5F1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||