

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50063300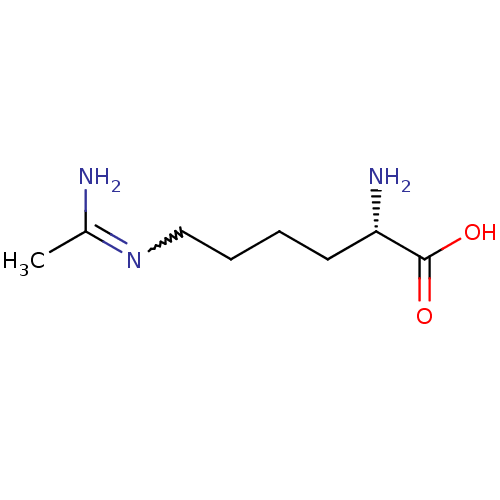 ((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Ability to inhibit conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by endothelial NOS (e NOS) from HUVEC cells | Bioorg Med Chem Lett 11: 1023-6 (2001) BindingDB Entry DOI: 10.7270/Q2K073JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50063300 ((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description In vitro inhibition of human endothelial nitric oxide synthase. | J Med Chem 47: 3320-3 (2004) Article DOI: 10.1021/jm031035n BindingDB Entry DOI: 10.7270/Q25M656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50063300 ((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Inhibition of human eNOS expressed in insect SF9 cells after 1 hr | Bioorg Med Chem Lett 18: 336-43 (2008) Article DOI: 10.1016/j.bmcl.2007.10.073 BindingDB Entry DOI: 10.7270/Q2H9961Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50063300 ((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood Curated by ChEMBL | Assay Description Inhibitory activity against human endothelial nitiric oxide synthase | J Med Chem 46: 913-6 (2003) Article DOI: 10.1021/jm0255926 BindingDB Entry DOI: 10.7270/Q2S75H3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50063300 ((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Rahway Curated by ChEMBL | Assay Description Inhibitory activity against endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 10: 1975-8 (2001) BindingDB Entry DOI: 10.7270/Q2319V30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50063300 ((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against Endothelial nitric oxide synthase | Bioorg Med Chem Lett 14: 4539-44 (2004) Article DOI: 10.1016/j.bmcl.2004.06.033 BindingDB Entry DOI: 10.7270/Q218377V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50063300 ((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Compound was evaluated for the Inhibition of endothelial isoform of Endothelial nitric oxide synthase (eNOS)enzyme | J Med Chem 41: 775-7 (1998) Article DOI: 10.1021/jm9706675 BindingDB Entry DOI: 10.7270/Q2VQ31TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50063300 ((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH Curated by ChEMBL | Assay Description Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting | Bioorg Med Chem Lett 21: 4228-32 (2011) Article DOI: 10.1016/j.bmcl.2011.05.073 BindingDB Entry DOI: 10.7270/Q2H996GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50063300 ((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition concentration against Endothelial nitric oxide synthase (eNOS) at 10 uM concentration | J Med Chem 47: 900-6 (2004) Article DOI: 10.1021/jm030348f BindingDB Entry DOI: 10.7270/Q23B60WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50063300 ((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human Endothelial nitric oxide synthase | J Med Chem 39: 669-72 (1996) Article DOI: 10.1021/jm950766n BindingDB Entry DOI: 10.7270/Q261110M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50063300 ((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description The concentration required for inhibition of Human endothelial nitric oxide synthase (eNOS) isoform | J Med Chem 45: 1686-9 (2002) BindingDB Entry DOI: 10.7270/Q2445N74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||