

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50258741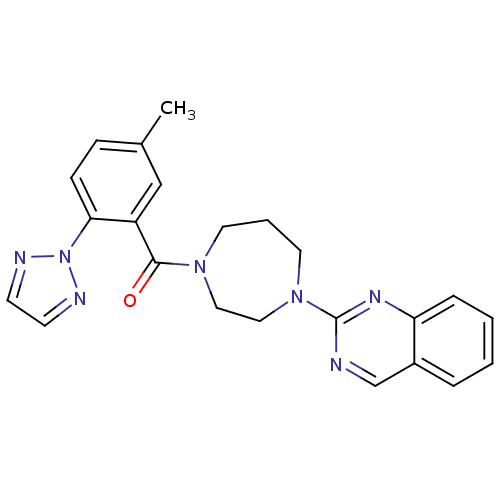 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX1 receptor (unknown origin) | Bioorg Med Chem Lett 19: 2997-3001 (2009) Article DOI: 10.1016/j.bmcl.2009.04.026 BindingDB Entry DOI: 10.7270/Q22J6BR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50258741 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50258741 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX1 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50258741 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX1R | Bioorg Med Chem Lett 20: 4201-5 (2010) Article DOI: 10.1016/j.bmcl.2010.05.047 BindingDB Entry DOI: 10.7270/Q25Q4X3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50258741 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50258741 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHO cells assessed as inhibition of orexin-A-induced intracellular calcium mobilization after 5 mins b... | J Med Chem 53: 5320-32 (2010) Article DOI: 10.1021/jm100541c BindingDB Entry DOI: 10.7270/Q29K4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50258741 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at OX1R (unknown origin) | J Med Chem 59: 504-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b00832 BindingDB Entry DOI: 10.7270/Q29C709W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50258741 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at OX1 receptor assessed as inhibition of calcium flux by FLIPR assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||