

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50109551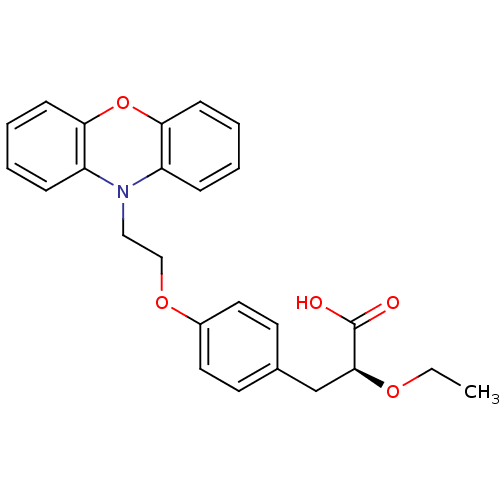 ((2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity for human peroxisome proliferator activated receptor alpha | J Med Chem 47: 4118-27 (2004) Article DOI: 10.1021/jm030631e BindingDB Entry DOI: 10.7270/Q2DR2TZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50109551 ((2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Receptor binding affinity of the compound to human Peroxisome proliferator activated receptor alpha against [3H]-NNC 0061-4655 radioligand | J Med Chem 46: 1306-17 (2003) Article DOI: 10.1021/jm021027r BindingDB Entry DOI: 10.7270/Q2P55MWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50109551 ((2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3.21E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro transactivation using receptor transactivation assay against hPPAR alpha | J Med Chem 45: 789-804 (2002) BindingDB Entry DOI: 10.7270/Q2445KSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50109551 ((2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3.21E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro activation of human peroxisome proliferator activated receptor alpha | Bioorg Med Chem Lett 13: 257-60 (2002) BindingDB Entry DOI: 10.7270/Q2FF3RQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50109551 ((2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Agonistic activity of the compound for Peroxisome proliferator activated receptor alpha | J Med Chem 46: 1306-17 (2003) Article DOI: 10.1021/jm021027r BindingDB Entry DOI: 10.7270/Q2P55MWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50109551 ((2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Transactivation of Homo sapiens (human) PPARalpha assessed as luciferase activity by reporter gene assay | Citation and Details Article DOI: 10.1007/s00044-011-9818-7 BindingDB Entry DOI: 10.7270/Q2R49TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50109551 ((2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 3.21E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Transactivation of GAL4-fused Homo sapiens (human) PPARalpha DNA binding domain expressed in African green monkey CV1 cells by luciferase reporter ge... | Citation and Details Article DOI: 10.1007/s00044-012-0003-4 BindingDB Entry DOI: 10.7270/Q280544F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50109551 ((2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Effective concentration against human peroxisome proliferator activated receptor alpha in Gal4 transactivation assay | J Med Chem 47: 4118-27 (2004) Article DOI: 10.1021/jm030631e BindingDB Entry DOI: 10.7270/Q2DR2TZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50109551 ((2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at GAL4-fused PPARalpha assessed as transcriptional activity by cell based assay | Bioorg Med Chem Lett 22: 7075-9 (2012) Article DOI: 10.1016/j.bmcl.2012.09.092 BindingDB Entry DOI: 10.7270/Q2HM59M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50109551 ((2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Ability to displace [3H]quipazine binding to 5-hydroxytryptamine 3 receptor sites in NG 108-15. | Bioorg Med Chem Lett 27: 3131-3134 (2017) Article DOI: 10.1016/j.bmcl.2017.05.037 BindingDB Entry DOI: 10.7270/Q20867QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50109551 ((2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 3.21E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Transactivation of GAL4-fused Homo sapiens (human) PPARalpha DNA binding domain expressed in African green monkey CV1 cells by luciferase reporter ge... | Citation and Details Article DOI: 10.1007/s00044-012-0003-4 BindingDB Entry DOI: 10.7270/Q280544F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||