

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50371235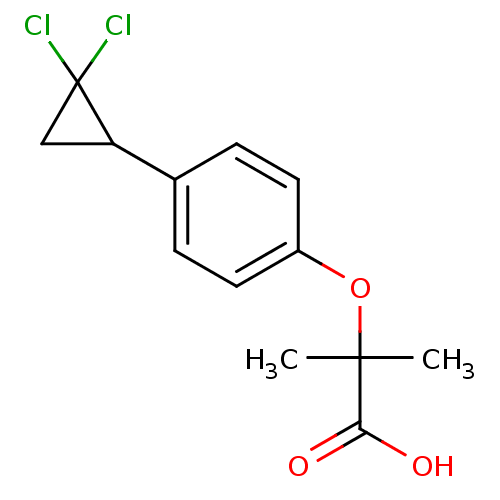 (CIPROFIBRATE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50371235 (CIPROFIBRATE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha LBD expressed in HepG2 cells after 20 hrs by luminescence assay | Bioorg Med Chem Lett 22: 2527-33 (2012) Article DOI: 10.1016/j.bmcl.2012.01.136 BindingDB Entry DOI: 10.7270/Q2P55PHF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Rattus norvegicus) | BDBM50371235 (CIPROFIBRATE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Curated by ChEMBL | Assay Description Agonist activity at rat PPARalpha in rat H4IIE cells assessed as gene induction | Bioorg Med Chem Lett 17: 6773-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.041 BindingDB Entry DOI: 10.7270/Q27945H6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||