

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471948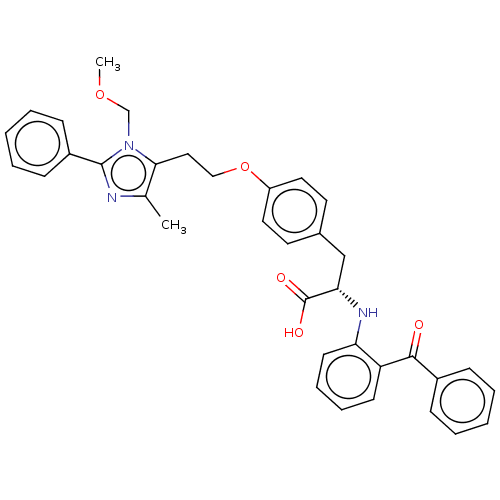 (CHEMBL149647) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition by 50% of in vitro binding to Peroxisome proliferator activated receptor gamma | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471948 (CHEMBL149647) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Ability to promote differentiation of C3H10T1/2 stem cells to adipocytes using lipogenesis assay mediated through activation of Peroxisome proliferat... | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50471948 (CHEMBL149647) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 81 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Tested functionally in vitro for inducing 50% of the maximum alkaline phosphate activity (Transactivation) against Peroxisome proliferator activated ... | J Med Chem 41: 5037-54 (1998) Article DOI: 10.1021/jm980413z BindingDB Entry DOI: 10.7270/Q2J38W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||