

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50394994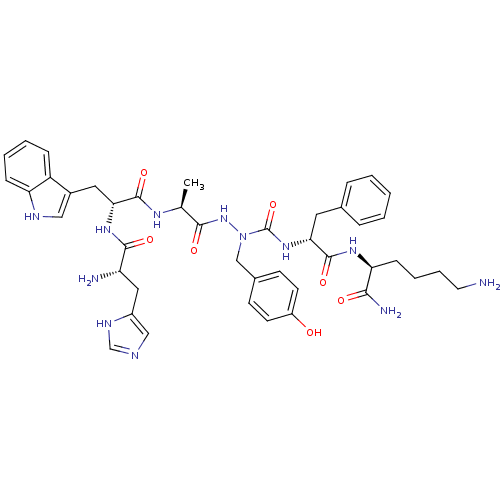 (CHEMBL2163484 | US9708370, DBG-178p) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of photoactivatable [125I]-Tyr-Bpa-Ala-hexarelin binding to CD36 in Sprague-Dawley rat heart membranes after 60 mins by SDS-PAGE analysis | J Med Chem 60: 9263-9274 (2017) Article DOI: 10.1021/acs.jmedchem.7b01209 BindingDB Entry DOI: 10.7270/Q2GF0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50394994 (CHEMBL2163484 | US9708370, DBG-178p) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50394994 (CHEMBL2163484 | US9708370, DBG-178p) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||