

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Procathepsin L (Homo sapiens (Human)) | BDBM210859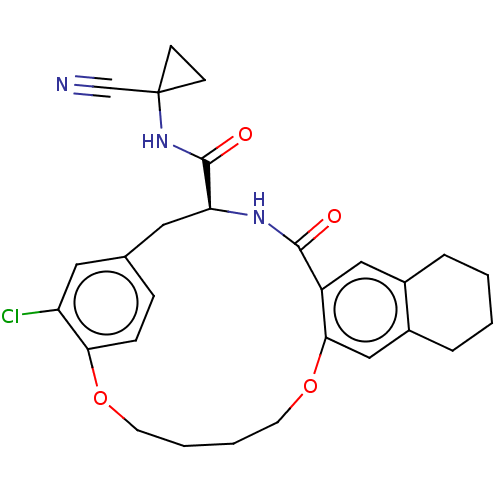 (US9290467, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210859 (US9290467, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 6.5 | n/a |
HOFFMANN—LA ROCHE INC. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore wh... | US Patent US9290467 (2016) BindingDB Entry DOI: 10.7270/Q2H130VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||