 Found 4 hits Enz. Inhib. hit(s) with Target = 'Prostacyclin receptor' and Ligand = 'BDBM50235379'
Found 4 hits Enz. Inhib. hit(s) with Target = 'Prostacyclin receptor' and Ligand = 'BDBM50235379' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235379
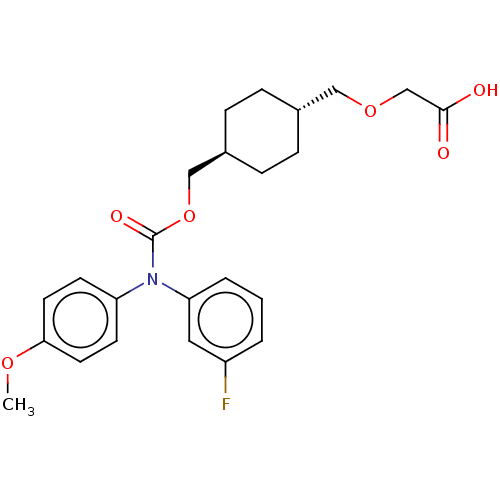
(CHEMBL3932106 | US10668033, Compound 55)Show SMILES COc1ccc(cc1)N(C(=O)OC[C@H]1CC[C@H](COCC(O)=O)CC1)c1cccc(F)c1 |r,wU:16.17,wD:13.13,(31.51,-37.36,;31.51,-35.82,;30.18,-35.05,;30.18,-33.51,;28.85,-32.74,;27.51,-33.51,;27.51,-35.05,;28.85,-35.82,;26.18,-32.74,;24.85,-33.51,;23.51,-32.74,;24.85,-35.05,;23.51,-35.82,;22.18,-35.05,;22.18,-33.51,;20.84,-32.74,;19.51,-33.51,;18.18,-32.74,;16.84,-33.51,;15.51,-32.74,;14.18,-33.51,;12.84,-32.74,;14.18,-35.05,;19.51,-35.05,;20.84,-35.82,;26.18,-31.2,;27.51,-30.43,;27.51,-28.89,;26.18,-28.12,;24.85,-28.89,;23.51,-28.12,;24.85,-30.43,)| Show InChI InChI=1S/C24H28FNO6/c1-30-22-11-9-20(10-12-22)26(21-4-2-3-19(25)13-21)24(29)32-15-18-7-5-17(6-8-18)14-31-16-23(27)28/h2-4,9-13,17-18H,5-8,14-16H2,1H3,(H,27,28)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235379

(CHEMBL3932106 | US10668033, Compound 55)Show SMILES COc1ccc(cc1)N(C(=O)OC[C@H]1CC[C@H](COCC(O)=O)CC1)c1cccc(F)c1 |r,wU:16.17,wD:13.13,(31.51,-37.36,;31.51,-35.82,;30.18,-35.05,;30.18,-33.51,;28.85,-32.74,;27.51,-33.51,;27.51,-35.05,;28.85,-35.82,;26.18,-32.74,;24.85,-33.51,;23.51,-32.74,;24.85,-35.05,;23.51,-35.82,;22.18,-35.05,;22.18,-33.51,;20.84,-32.74,;19.51,-33.51,;18.18,-32.74,;16.84,-33.51,;15.51,-32.74,;14.18,-33.51,;12.84,-32.74,;14.18,-35.05,;19.51,-35.05,;20.84,-35.82,;26.18,-31.2,;27.51,-30.43,;27.51,-28.89,;26.18,-28.12,;24.85,-28.89,;23.51,-28.12,;24.85,-30.43,)| Show InChI InChI=1S/C24H28FNO6/c1-30-22-11-9-20(10-12-22)26(21-4-2-3-19(25)13-21)24(29)32-15-18-7-5-17(6-8-18)14-31-16-23(27)28/h2-4,9-13,17-18H,5-8,14-16H2,1H3,(H,27,28)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | n/a | n/a | 5.14 | n/a | n/a | n/a | n/a |
ARENA PHARMACEUTICALS, INC.
US Patent
| Assay Description
Compounds were screened for agonists of the human prostacyclin (PGI2) receptor using the HTRF assay for direct cAMP measurement (Gabriel et al., ASSA... |
US Patent US10668033 (2020)
BindingDB Entry DOI: 10.7270/Q2ZW1PX1 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235379

(CHEMBL3932106 | US10668033, Compound 55)Show SMILES COc1ccc(cc1)N(C(=O)OC[C@H]1CC[C@H](COCC(O)=O)CC1)c1cccc(F)c1 |r,wU:16.17,wD:13.13,(31.51,-37.36,;31.51,-35.82,;30.18,-35.05,;30.18,-33.51,;28.85,-32.74,;27.51,-33.51,;27.51,-35.05,;28.85,-35.82,;26.18,-32.74,;24.85,-33.51,;23.51,-32.74,;24.85,-35.05,;23.51,-35.82,;22.18,-35.05,;22.18,-33.51,;20.84,-32.74,;19.51,-33.51,;18.18,-32.74,;16.84,-33.51,;15.51,-32.74,;14.18,-33.51,;12.84,-32.74,;14.18,-35.05,;19.51,-35.05,;20.84,-35.82,;26.18,-31.2,;27.51,-30.43,;27.51,-28.89,;26.18,-28.12,;24.85,-28.89,;23.51,-28.12,;24.85,-30.43,)| Show InChI InChI=1S/C24H28FNO6/c1-30-22-11-9-20(10-12-22)26(21-4-2-3-19(25)13-21)24(29)32-15-18-7-5-17(6-8-18)14-31-16-23(27)28/h2-4,9-13,17-18H,5-8,14-16H2,1H3,(H,27,28)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(RAT) | BDBM50235379

(CHEMBL3932106 | US10668033, Compound 55)Show SMILES COc1ccc(cc1)N(C(=O)OC[C@H]1CC[C@H](COCC(O)=O)CC1)c1cccc(F)c1 |r,wU:16.17,wD:13.13,(31.51,-37.36,;31.51,-35.82,;30.18,-35.05,;30.18,-33.51,;28.85,-32.74,;27.51,-33.51,;27.51,-35.05,;28.85,-35.82,;26.18,-32.74,;24.85,-33.51,;23.51,-32.74,;24.85,-35.05,;23.51,-35.82,;22.18,-35.05,;22.18,-33.51,;20.84,-32.74,;19.51,-33.51,;18.18,-32.74,;16.84,-33.51,;15.51,-32.74,;14.18,-33.51,;12.84,-32.74,;14.18,-35.05,;19.51,-35.05,;20.84,-35.82,;26.18,-31.2,;27.51,-30.43,;27.51,-28.89,;26.18,-28.12,;24.85,-28.89,;23.51,-28.12,;24.85,-30.43,)| Show InChI InChI=1S/C24H28FNO6/c1-30-22-11-9-20(10-12-22)26(21-4-2-3-19(25)13-21)24(29)32-15-18-7-5-17(6-8-18)14-31-16-23(27)28/h2-4,9-13,17-18H,5-8,14-16H2,1H3,(H,27,28)/t17-,18- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant rat IP receptor expressed in CHO-K1 cells assessed as increase in intracellular cAMP level after 1 hr incubation by H... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



