 Found 5 hits Enz. Inhib. hit(s) with Target = 'Prostaglandin G/H synthase 2' and Ligand = 'BDBM50110164'
Found 5 hits Enz. Inhib. hit(s) with Target = 'Prostaglandin G/H synthase 2' and Ligand = 'BDBM50110164' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110164
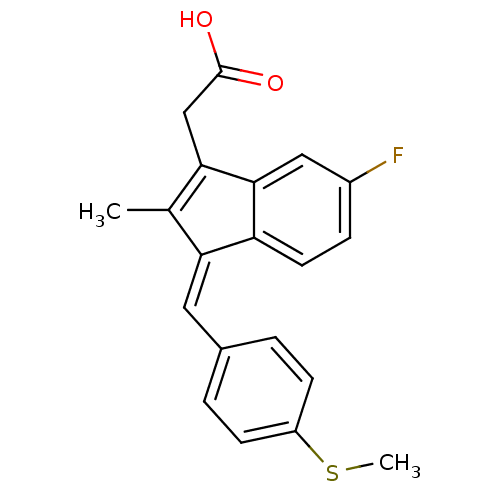
((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...)Show SMILES CSc1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:9| Show InChI InChI=1S/C20H17FO2S/c1-12-17(9-13-3-6-15(24-2)7-4-13)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Bartholomew's and the Royal London School of Medicine and Dentistry
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 96: 7563-8 (1999)
Article DOI: 10.1073/pnas.96.13.7563
BindingDB Entry DOI: 10.7270/Q21G0JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110164

((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...)Show SMILES CSc1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:9| Show InChI InChI=1S/C20H17FO2S/c1-12-17(9-13-3-6-15(24-2)7-4-13)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Bartholomew's and the Royal London School of Medicine and Dentistry
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 96: 7563-8 (1999)
Article DOI: 10.1073/pnas.96.13.7563
BindingDB Entry DOI: 10.7270/Q21G0JT4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110164

((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...)Show SMILES CSc1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:9| Show InChI InChI=1S/C20H17FO2S/c1-12-17(9-13-3-6-15(24-2)7-4-13)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110164

((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...)Show SMILES CSc1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:9| Show InChI InChI=1S/C20H17FO2S/c1-12-17(9-13-3-6-15(24-2)7-4-13)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Institute of Chemical Biology
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Med Chem 55: 2287-300 (2012)
Article DOI: 10.1021/jm201528b
BindingDB Entry DOI: 10.7270/Q2WW7JQB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110164

((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...)Show SMILES CSc1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:9| Show InChI InChI=1S/C20H17FO2S/c1-12-17(9-13-3-6-15(24-2)7-4-13)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition by microplate reader... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113542
BindingDB Entry DOI: 10.7270/Q2NG4VGG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data