

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM205427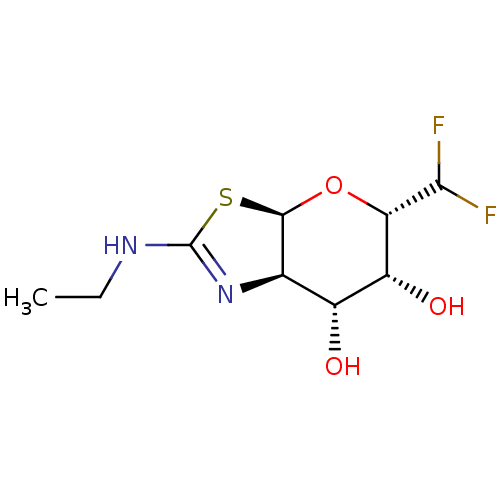 (US9243020, 29 | US9815861, Example 29) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics, Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9815861 (2017) BindingDB Entry DOI: 10.7270/Q2R78HJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM205427 (US9243020, 29 | US9815861, Example 29) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 164 | -38.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9243020 (2016) BindingDB Entry DOI: 10.7270/Q2542MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||