

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase receptor Ret [658-1114,V804M] (Homo sapiens (Human)) | BDBM305355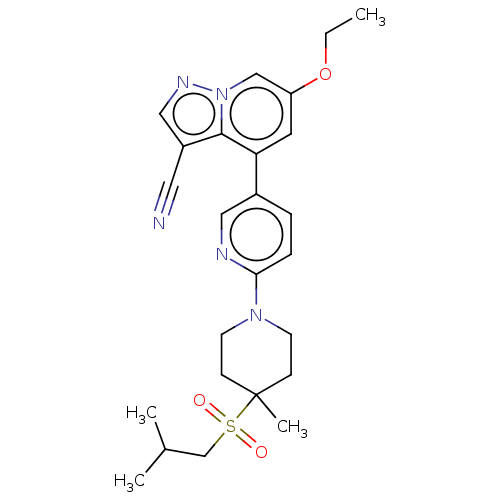 (US10144734, Example 371 | US10172845, Example 371 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Array Biopharma Inc. US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF KinEASE-TK assay techn... | US Patent US10441581 (2019) BindingDB Entry DOI: 10.7270/Q2DZ0BP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret [658-1114,V804M] (Homo sapiens (Human)) | BDBM305355 (US10144734, Example 371 | US10172845, Example 371 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc. US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit wildtype and V804M mutant RET kinase using CisBio's HTRF® KinEASE-TK assay tech... | US Patent US10172845 (2019) BindingDB Entry DOI: 10.7270/Q2M90BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||