

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyruvate kinase PKLR (Rattus norvegicus) | BDBM50367054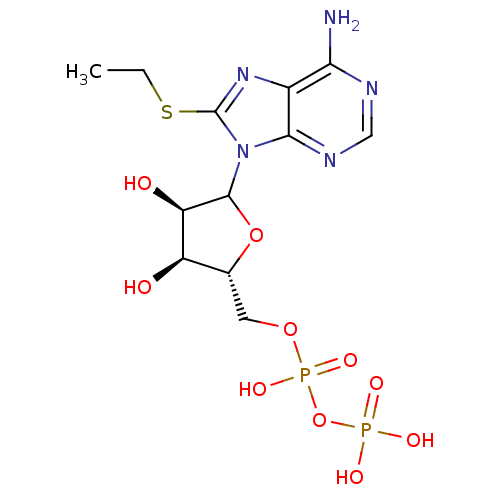 (CHEMBL606476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat kidney pyruvate kinase (PK-K) at 6.7 mM | J Med Chem 25: 1184-8 (1983) BindingDB Entry DOI: 10.7270/Q21N81PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyruvate kinase PKLR (Rattus norvegicus) | BDBM50367054 (CHEMBL606476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 8.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant for inhibition of pyruvate kinase obtained from rat liver (PK-L) was determined using ADP as competitive inhibitor | J Med Chem 25: 1184-8 (1983) BindingDB Entry DOI: 10.7270/Q21N81PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||