

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099692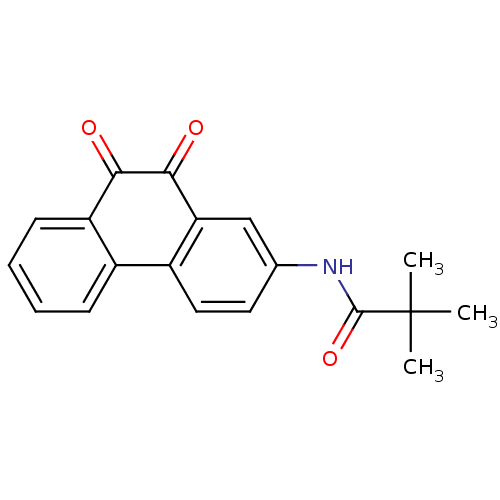 (CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against the cytosolic portion of CD45 protein-tyrosine phosphatase using pNPP as the substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099692 (CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
United States Army Medical Research Institute of Infectious Diseases | Assay Description Protein phosphatases were purchased from Upstate Biotechnology (Lake Placid, NY). | J Biol Chem 284: 12874-85 (2009) Article DOI: 10.1074/jbc.M809633200 BindingDB Entry DOI: 10.7270/Q2J67FJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase C (Homo sapiens (Human)) | BDBM50099692 (CHEMBL51314 | N-(9,10-Dioxo-9,10-dihydro-phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against CD45 protein-tyrosine phosphatase using lck-10 mer as substrate | J Med Chem 44: 1777-93 (2001) BindingDB Entry DOI: 10.7270/Q2X34WQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||