 Found 3 hits Enz. Inhib. hit(s) with Target = 'Retinoic acid receptor alpha' and Ligand = 'BDBM50061625'
Found 3 hits Enz. Inhib. hit(s) with Target = 'Retinoic acid receptor alpha' and Ligand = 'BDBM50061625' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50061625
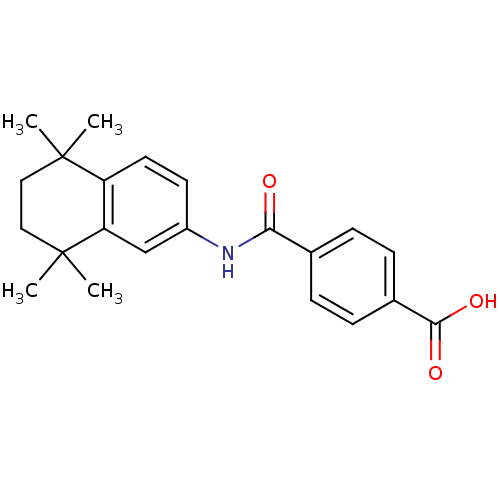
(4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(NC(=O)c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)23-19(24)14-5-7-15(8-6-14)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoic acid receptor-alpha |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50061625

(4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(NC(=O)c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)23-19(24)14-5-7-15(8-6-14)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic Acid Receptor alpha (RAR alpha) |
J Med Chem 43: 409-19 (2000)
BindingDB Entry DOI: 10.7270/Q28S4P4M |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50061625

(4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(NC(=O)c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)23-19(24)14-5-7-15(8-6-14)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Aristotle University of Thessaloniki
Curated by ChEMBL
| Assay Description
Agonist activity at RARalpha |
J Med Chem 53: 6779-810 (2010)
Article DOI: 10.1021/jm100189a
BindingDB Entry DOI: 10.7270/Q29C6XPM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data