

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM402003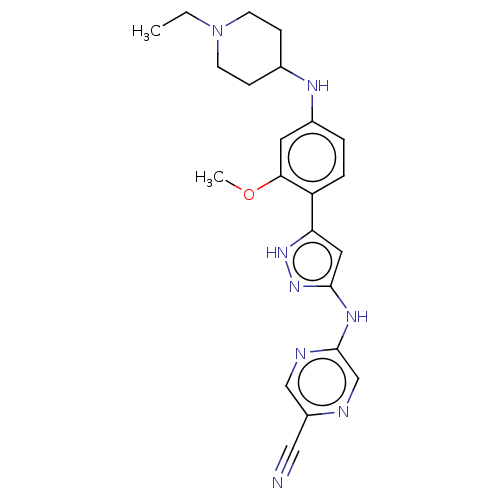 (5-[[5-[4-[(1-ethyl-4-piperidyl)amino]-2-methoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
SENTINEL ONCOLOGY LIMITED US Patent | Assay Description Base Reaction buffer: 20 mM Hepes (pH 7.5), 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/ml BSA, 0.1 mM Na3VO4, 2 mM DTT, 1% DMSO Required co... | US Patent US10973817 (2021) BindingDB Entry DOI: 10.7270/Q2K93BNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM402003 (5-[[5-[4-[(1-ethyl-4-piperidyl)amino]-2-methoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL Rome | Assay Description Chk-1 Kinase Inhibiting ActivityThe compounds of the invention were tested for activity against Chk-1 kinase using the materials and protocols set ou... | J Med Chem 52: 5217-27 (2009) BindingDB Entry DOI: 10.7270/Q2NK3HCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||