

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451770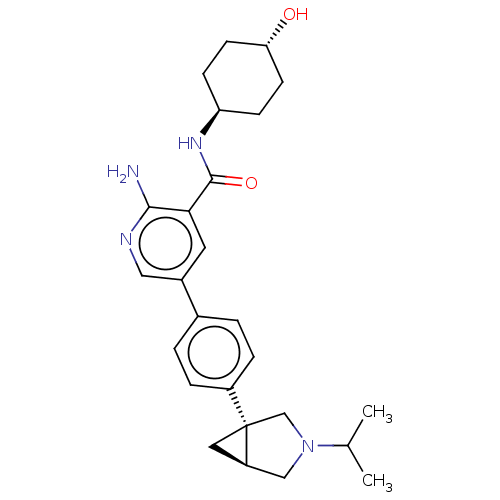 (US10710980, Example 2 | US10947218, Example 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for wild-type ALK2 (aa172-499), ALK2 FOP mutant (aa172-499 R20... | US Patent US10947218 (2021) BindingDB Entry DOI: 10.7270/Q24F1TVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 [166-493] (Homo sapiens (Human)) | BDBM451770 (US10710980, Example 2 | US10947218, Example 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description In the applied method, this separation takes place inside a chip that contains a complex capillary system for simultaneous analysis of 12 samples (“1... | US Patent US10710980 (2020) BindingDB Entry DOI: 10.7270/Q2DF6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||