

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM368413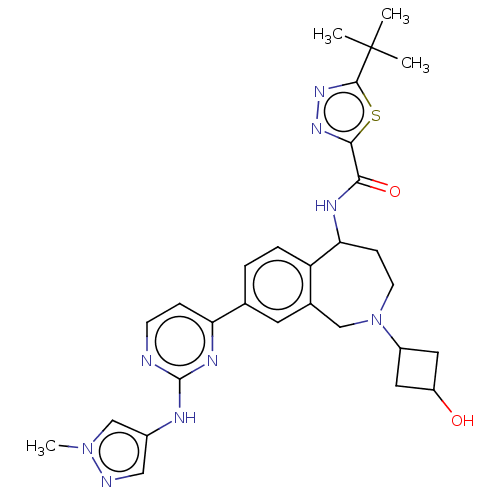 (3-(tert-butyl)-N-(2-(3- hydroxycyclobutyl)-8-(2- (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 505 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience | Assay Description The purpose of the BTK in vitro assay is to determine compound potency against BTK through the measurement of IC50. Compound inhibition is measured a... | J Med Chem 51: 1730-9 (2008) BindingDB Entry DOI: 10.7270/Q25M681D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||