 Found 8 hits Enz. Inhib. hit(s) with Target = 'Tyrosine-protein kinase BTK' and Ligand = 'BDBM50459553'
Found 8 hits Enz. Inhib. hit(s) with Target = 'Tyrosine-protein kinase BTK' and Ligand = 'BDBM50459553' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50459553
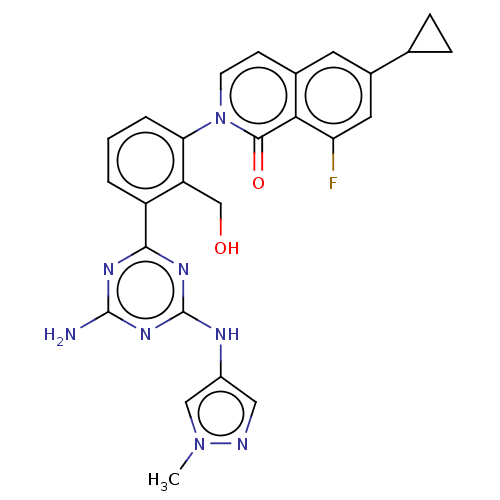
(CHEMBL4209441)Show SMILES Cn1cc(Nc2nc(N)nc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cn1 Show InChI InChI=1S/C26H23FN8O2/c1-34-12-17(11-29-34)30-26-32-23(31-25(28)33-26)18-3-2-4-21(19(18)13-36)35-8-7-15-9-16(14-5-6-14)10-20(27)22(15)24(35)37/h2-4,7-12,14,36H,5-6,13H2,1H3,(H3,28,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal DYKDDDDK tagged biotinylated unactivated human recombinant BTK using FITC-labeled Srctide peptide substrate by by mobility s... |
J Med Chem 61: 8917-8933 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01147
BindingDB Entry DOI: 10.7270/Q2ZS305M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50459553

(CHEMBL4209441)Show SMILES Cn1cc(Nc2nc(N)nc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cn1 Show InChI InChI=1S/C26H23FN8O2/c1-34-12-17(11-29-34)30-26-32-23(31-25(28)33-26)18-3-2-4-21(19(18)13-36)35-8-7-15-9-16(14-5-6-14)10-20(27)22(15)24(35)37/h2-4,7-12,14,36H,5-6,13H2,1H3,(H3,28,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50459553

(CHEMBL4209441)Show SMILES Cn1cc(Nc2nc(N)nc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cn1 Show InChI InChI=1S/C26H23FN8O2/c1-34-12-17(11-29-34)30-26-32-23(31-25(28)33-26)18-3-2-4-21(19(18)13-36)35-8-7-15-9-16(14-5-6-14)10-20(27)22(15)24(35)37/h2-4,7-12,14,36H,5-6,13H2,1H3,(H3,28,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal DYKDDDDK tagged biotinylated activated human recombinant BTK using FITC-labeled Srctide peptide substrate by mobility shift ... |
J Med Chem 61: 8917-8933 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01147
BindingDB Entry DOI: 10.7270/Q2ZS305M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50459553

(CHEMBL4209441)Show SMILES Cn1cc(Nc2nc(N)nc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cn1 Show InChI InChI=1S/C26H23FN8O2/c1-34-12-17(11-29-34)30-26-32-23(31-25(28)33-26)18-3-2-4-21(19(18)13-36)35-8-7-15-9-16(14-5-6-14)10-20(27)22(15)24(35)37/h2-4,7-12,14,36H,5-6,13H2,1H3,(H3,28,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human PBMC assessed as reduction in anti-IgM-stimulated B-cell activation by measuring CD86 expression |
J Med Chem 61: 8917-8933 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01147
BindingDB Entry DOI: 10.7270/Q2ZS305M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50459553

(CHEMBL4209441)Show SMILES Cn1cc(Nc2nc(N)nc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cn1 Show InChI InChI=1S/C26H23FN8O2/c1-34-12-17(11-29-34)30-26-32-23(31-25(28)33-26)18-3-2-4-21(19(18)13-36)35-8-7-15-9-16(14-5-6-14)10-20(27)22(15)24(35)37/h2-4,7-12,14,36H,5-6,13H2,1H3,(H3,28,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of anti-IgM-stimulated BTK phosphorylation at Tyr223 in human Ramos cells pre-incubated for 1 hr followed by anti-IgM stimulation for 10 m... |
J Med Chem 61: 8917-8933 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01147
BindingDB Entry DOI: 10.7270/Q2ZS305M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50459553

(CHEMBL4209441)Show SMILES Cn1cc(Nc2nc(N)nc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cn1 Show InChI InChI=1S/C26H23FN8O2/c1-34-12-17(11-29-34)30-26-32-23(31-25(28)33-26)18-3-2-4-21(19(18)13-36)35-8-7-15-9-16(14-5-6-14)10-20(27)22(15)24(35)37/h2-4,7-12,14,36H,5-6,13H2,1H3,(H3,28,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of anti-IgM-stimulated BTK phosphorylation at Tyr551 in human Ramos cells pre-incubated for 1 hr followed by anti-IgM stimulation for 10 m... |
J Med Chem 61: 8917-8933 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01147
BindingDB Entry DOI: 10.7270/Q2ZS305M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50459553

(CHEMBL4209441)Show SMILES Cn1cc(Nc2nc(N)nc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cn1 Show InChI InChI=1S/C26H23FN8O2/c1-34-12-17(11-29-34)30-26-32-23(31-25(28)33-26)18-3-2-4-21(19(18)13-36)35-8-7-15-9-16(14-5-6-14)10-20(27)22(15)24(35)37/h2-4,7-12,14,36H,5-6,13H2,1H3,(H3,28,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of anti-IgM-stimulated BTK-mediated PLCgamma2 phosphorylation at Tyr1217 in human Ramos cells pre-incubated for 1 hr followed by anti-IgM ... |
J Med Chem 61: 8917-8933 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01147
BindingDB Entry DOI: 10.7270/Q2ZS305M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50459553

(CHEMBL4209441)Show SMILES Cn1cc(Nc2nc(N)nc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cn1 Show InChI InChI=1S/C26H23FN8O2/c1-34-12-17(11-29-34)30-26-32-23(31-25(28)33-26)18-3-2-4-21(19(18)13-36)35-8-7-15-9-16(14-5-6-14)10-20(27)22(15)24(35)37/h2-4,7-12,14,36H,5-6,13H2,1H3,(H3,28,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK phosphorylation in human whole blood assessed as reduction in BTK phosphorylation incubated for 30 mins |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data