

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V1a receptor (RAT) | BDBM50029649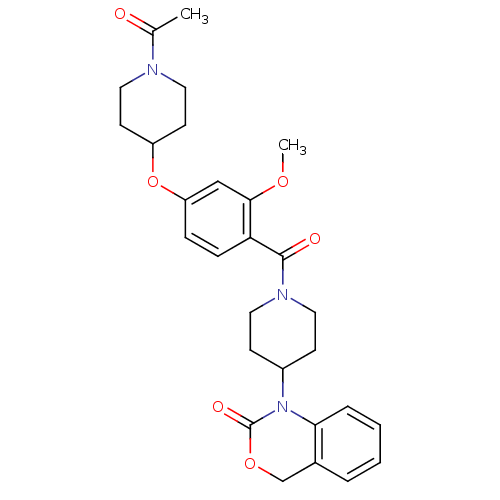 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V1a receptor in rat liver tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in rat liver | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | <2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Compound was tested for its ability to displace vasopressin from human Vasopressin V1a receptor | Bioorg Med Chem Lett 12: 1405-11 (2002) BindingDB Entry DOI: 10.7270/Q2C24VRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Vasopressin V1a receptor by using functional assay | Bioorg Med Chem Lett 9: 1311-6 (1999) BindingDB Entry DOI: 10.7270/Q23N22K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated by measuring the displacement of [3H]-AVP (arginine vasopressin) from specific binding sites in human platelets | J Med Chem 38: 4634-6 (1995) BindingDB Entry DOI: 10.7270/Q2ZW1JXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from vasopressin V1a receptor in human liver tissue | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029649 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
UMR 7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Activity at human vasopressin V1a receptor expressed in CHO cells by NFAT-luciferase gene reporter assay | J Med Chem 53: 1546-62 (2010) Article DOI: 10.1021/jm901084f BindingDB Entry DOI: 10.7270/Q2FX7BD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||