

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM11340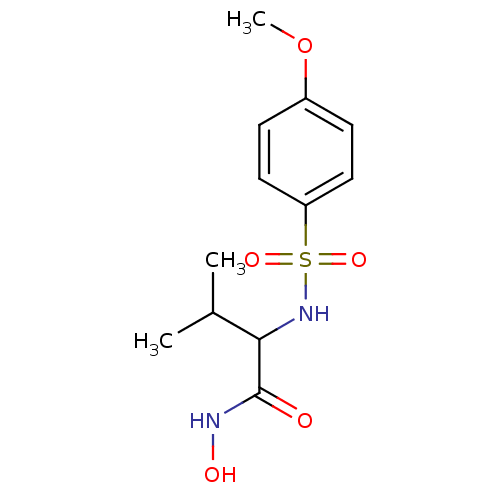 (Hydroxamate 21 | N-hydroxy-2-[(4-methoxybenzene)su...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | -10.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11340 (Hydroxamate 21 | N-hydroxy-2-[(4-methoxybenzene)su...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11340 (Hydroxamate 21 | N-hydroxy-2-[(4-methoxybenzene)su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11340 (Hydroxamate 21 | N-hydroxy-2-[(4-methoxybenzene)su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description Initial rates for the hydrolysis of the thioester substrate AcProLeuGly-S-LeuLeuGlyOEt, coupled to the reaction with 5,5-dithiobis(2-nitrobenzoic aci... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase ColG (Clostridium histolyticum) | BDBM11340 (Hydroxamate 21 | N-hydroxy-2-[(4-methoxybenzene)su...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description The rate of hydrolysis was determined from the change in absorbance at 324 nm using an extinction coefficient, 24700 M-1 cm-1 for FALGPA. Initial vel... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM11340 (Hydroxamate 21 | N-hydroxy-2-[(4-methoxybenzene)su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description Initial rates for the hydrolysis of the thioester substrate AcProLeuGly-S-LeuLeuGlyOEt, coupled to the reaction with 5,5-dithiobis(2-nitrobenzoic aci... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM11340 (Hydroxamate 21 | N-hydroxy-2-[(4-methoxybenzene)su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description Initial rates for the hydrolysis of the thioester substrate AcProLeuGly-S-LeuLeuGlyOEt, coupled to the reaction with 5,5-dithiobis(2-nitrobenzoic aci... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM11340 (Hydroxamate 21 | N-hydroxy-2-[(4-methoxybenzene)su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description Initial rates for the hydrolysis of the thioester substrate AcProLeuGly-S-LeuLeuGlyOEt, coupled to the reaction with 5,5-dithiobis(2-nitrobenzoic aci... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||