

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50155838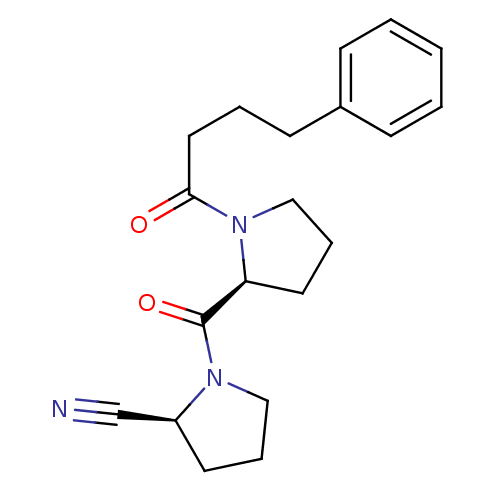 ((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Inhibition of prolyl oligopeptidase (unknown origin) | Eur J Med Chem 79: 436-45 (2014) Article DOI: 10.1016/j.ejmech.2014.04.014 BindingDB Entry DOI: 10.7270/Q2G162BW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135635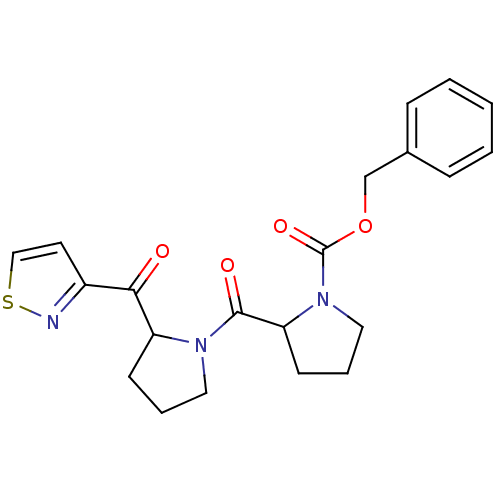 (2-[2-(Isothiazole-3-carbonyl)-pyrrolidine-1-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135637 (1-{(S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038879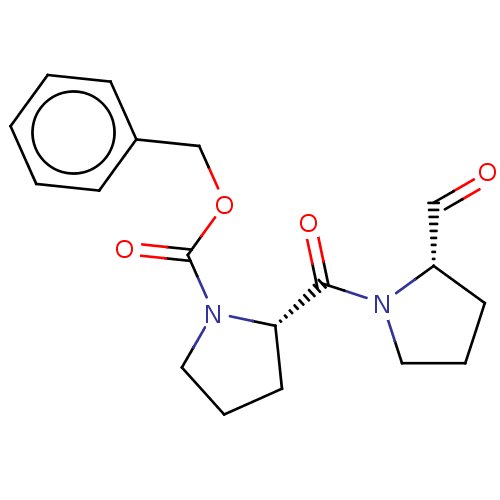 ((S)-2-(2-Formyl-pyrrolidine-1-carbonyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of recombinant human POP assessed as affinity constant of second step of inhibition pre-incubated for 2 hrs before addition of ZGP-pNA sub... | J Med Chem 62: 7874-7884 (2019) Article DOI: 10.1021/acs.jmedchem.9b00642 BindingDB Entry DOI: 10.7270/Q2FB5682 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135634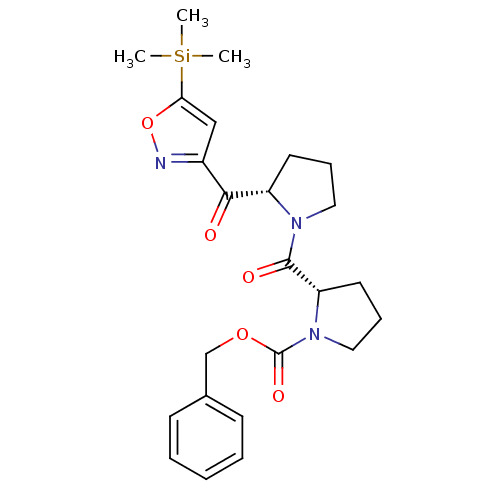 ((S)-2-[(S)-2-(5-Trimethylsilanyl-isoxazole-3-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50431227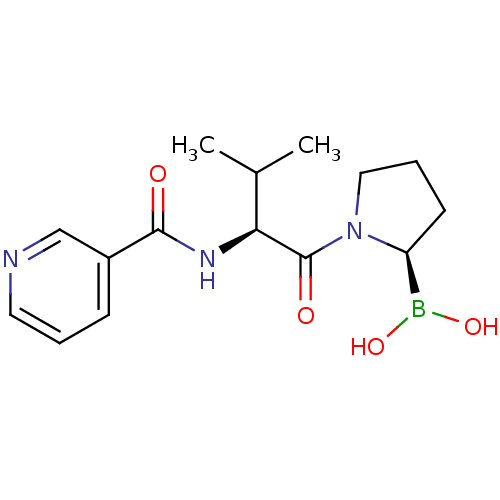 (CHEMBL2333024) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of human PREP using Suc-GP-AMC as substrate by Lineweaver-Burk plot analysis | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180679 (CHEMBL3817958) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP by tight binding based Morrison equation analysis | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50155838 ((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP by tight binding based Morrison equation analysis | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50155838 ((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50051539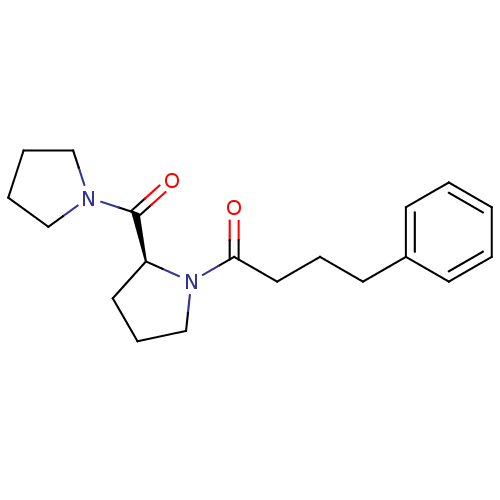 ((S)-4-phenyl-1-(2-(pyrrolidine-1-carbonyl)pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Inhibition of prolyl oligopeptidase (unknown origin) | Eur J Med Chem 79: 436-45 (2014) Article DOI: 10.1016/j.ejmech.2014.04.014 BindingDB Entry DOI: 10.7270/Q2G162BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180679 (CHEMBL3817958) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038879 ((S)-2-(2-Formyl-pyrrolidine-1-carbonyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of recombinant human POP pre-incubated for 30 mins before addition of ZGP-pNA substrate by absorbance assay | J Med Chem 62: 7874-7884 (2019) Article DOI: 10.1021/acs.jmedchem.9b00642 BindingDB Entry DOI: 10.7270/Q2FB5682 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50316818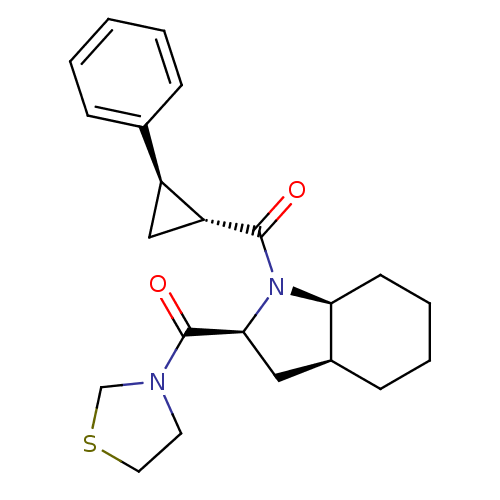 (((2S,3aS,7aS)-1-((1R,2R)-2-phenylcyclopropanecarbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP | J Med Chem 53: 3423-38 (2010) Article DOI: 10.1021/jm901104g BindingDB Entry DOI: 10.7270/Q261119N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50316818 (((2S,3aS,7aS)-1-((1R,2R)-2-phenylcyclopropanecarbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP using Z-Gly-Pro-7-AMC substrate | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50200728 ((R)-1-(4-oxo-4-phenylbutanoyl)pyrrolidin-2-ylboron...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibitory constant against POP | Bioorg Med Chem Lett 17: 1438-42 (2007) Article DOI: 10.1016/j.bmcl.2006.11.072 BindingDB Entry DOI: 10.7270/Q2VH5NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50200729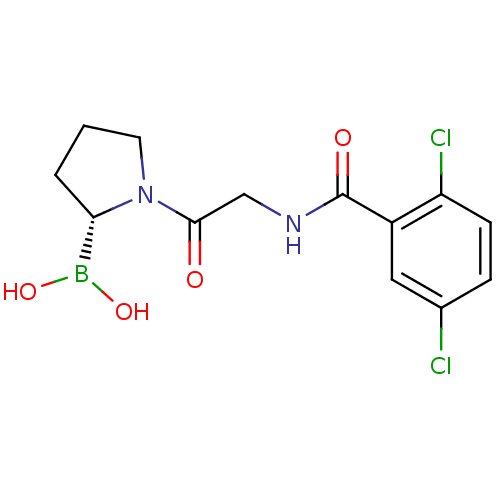 ((R)-1-(2-(2,5-dichlorobenzamido)acetyl)pyrrolidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of recombinant POP | J Med Chem 53: 3423-38 (2010) Article DOI: 10.1021/jm901104g BindingDB Entry DOI: 10.7270/Q261119N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50200729 ((R)-1-(2-(2,5-dichlorobenzamido)acetyl)pyrrolidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibitory constant against POP | Bioorg Med Chem Lett 17: 1438-42 (2007) Article DOI: 10.1016/j.bmcl.2006.11.072 BindingDB Entry DOI: 10.7270/Q2VH5NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50200726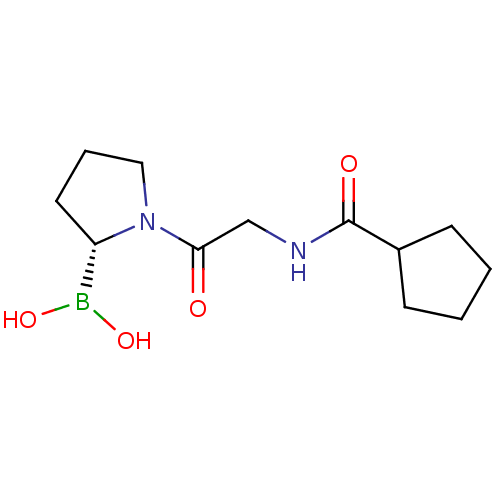 ((R)-1-(2-(cyclopentanecarboxamido)acetyl)pyrrolidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibitory constant against POP | Bioorg Med Chem Lett 17: 1438-42 (2007) Article DOI: 10.1016/j.bmcl.2006.11.072 BindingDB Entry DOI: 10.7270/Q2VH5NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50200721 ((R)-1-(2-(cyclohexanecarboxamido)acetyl)pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibitory constant against POP | Bioorg Med Chem Lett 17: 1438-42 (2007) Article DOI: 10.1016/j.bmcl.2006.11.072 BindingDB Entry DOI: 10.7270/Q2VH5NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50200723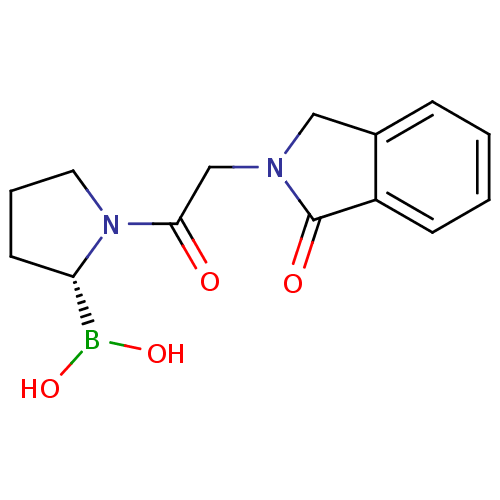 ((R)-1-(2-(1-oxoisoindolin-2-yl)acetyl)pyrrolidin-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibitory constant against POP | Bioorg Med Chem Lett 17: 1438-42 (2007) Article DOI: 10.1016/j.bmcl.2006.11.072 BindingDB Entry DOI: 10.7270/Q2VH5NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of PKD2 ( assessed as residual activity at 1 uM ) by TR-FRET assay | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50508977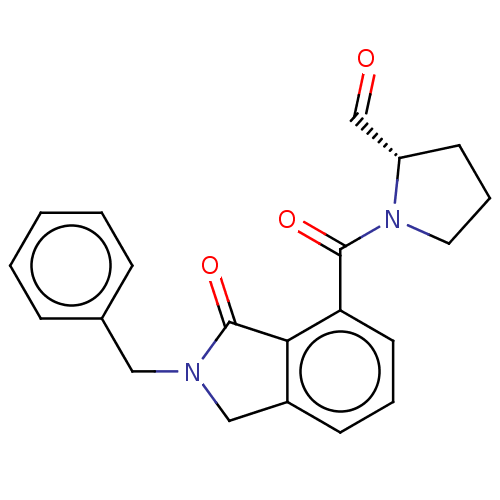 (CHEMBL4451993) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of recombinant human POP assessed as affinity constant of second step of inhibition pre-incubated for 2 hrs before addition of ZGP-pNA sub... | J Med Chem 62: 7874-7884 (2019) Article DOI: 10.1021/acs.jmedchem.9b00642 BindingDB Entry DOI: 10.7270/Q2FB5682 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human prolyl endopeptidase | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50508977 (CHEMBL4451993) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of recombinant human POP pre-incubated for 30 mins before addition of ZGP-pNA substrate by absorbance assay | J Med Chem 62: 7874-7884 (2019) Article DOI: 10.1021/acs.jmedchem.9b00642 BindingDB Entry DOI: 10.7270/Q2FB5682 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038879 ((S)-2-(2-Formyl-pyrrolidine-1-carbonyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Warwick Curated by ChEMBL | Assay Description Inhibition of prolyl oligopeptidase | Bioorg Med Chem 18: 4775-82 (2011) Article DOI: 10.1016/j.bmc.2010.05.012 BindingDB Entry DOI: 10.7270/Q2000319 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50200720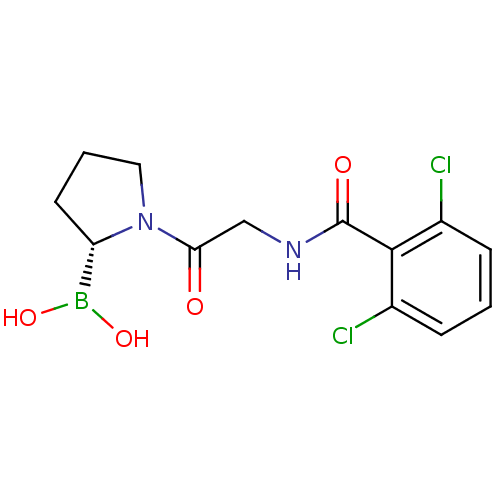 ((R)-1-(2-(2,6-dichlorobenzamido)acetyl)pyrrolidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibitory constant against POP | Bioorg Med Chem Lett 17: 1438-42 (2007) Article DOI: 10.1016/j.bmcl.2006.11.072 BindingDB Entry DOI: 10.7270/Q2VH5NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50200732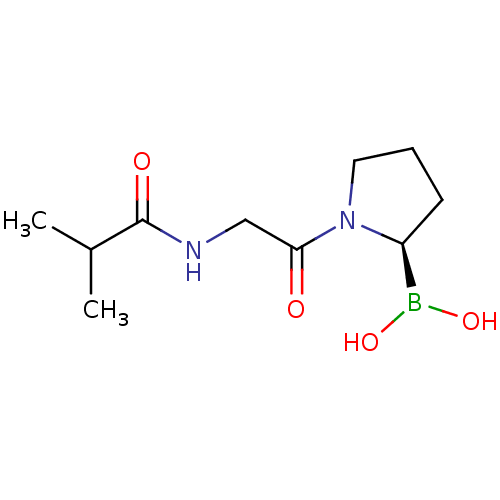 ((R)-1-(2-isobutyramidoacetyl)pyrrolidin-2-ylboroni...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibitory constant against POP | Bioorg Med Chem Lett 17: 1438-42 (2007) Article DOI: 10.1016/j.bmcl.2006.11.072 BindingDB Entry DOI: 10.7270/Q2VH5NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50504804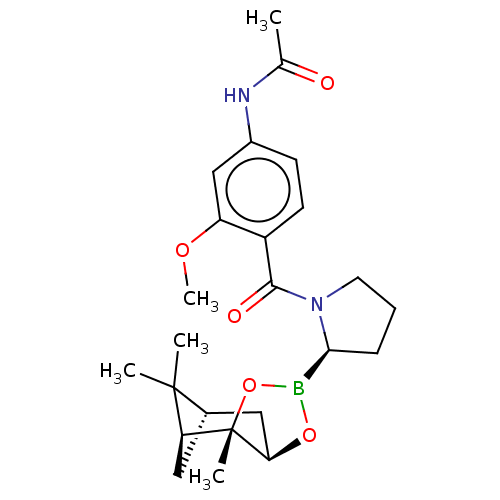 (CHEMBL4559324) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged POP (2 to 710 residues) expressed in SF21 cells using Z-Gly-Pro-AMC as substrate incubated for ... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111783 BindingDB Entry DOI: 10.7270/Q2CV4N01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50374364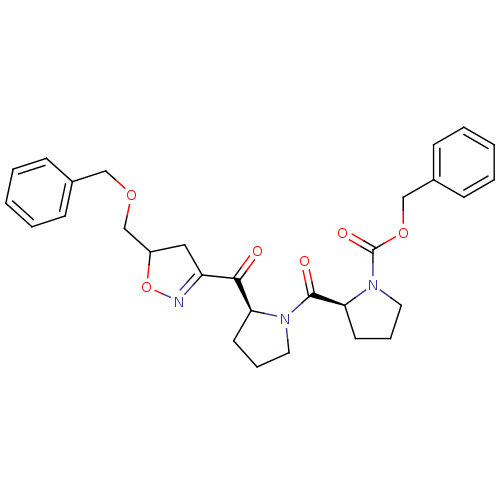 (CHEMBL273161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human prolyl endopeptidase | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50200725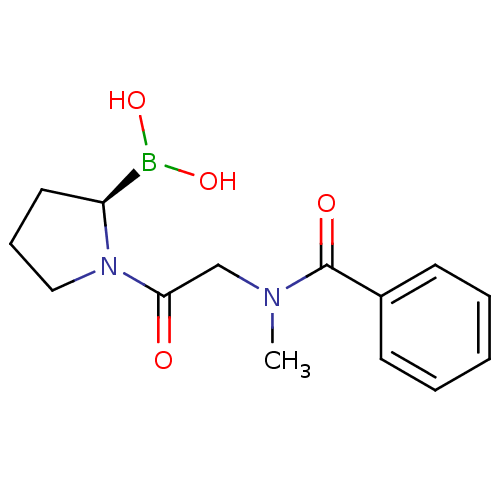 ((R)-1-(2-(N-methylbenzamido)acetyl)pyrrolidin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibitory constant against POP | Bioorg Med Chem Lett 17: 1438-42 (2007) Article DOI: 10.1016/j.bmcl.2006.11.072 BindingDB Entry DOI: 10.7270/Q2VH5NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135636 ((S)-2-[(S)-2-(5-Cyano-isoxazole-3-carbonyl)-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038879 ((S)-2-(2-Formyl-pyrrolidine-1-carbonyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of recombinant human POP assessed as affinity constant of first step of the binding event pre-incubated for 2 hrs before addition of ZGP-p... | J Med Chem 62: 7874-7884 (2019) Article DOI: 10.1021/acs.jmedchem.9b00642 BindingDB Entry DOI: 10.7270/Q2FB5682 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50504801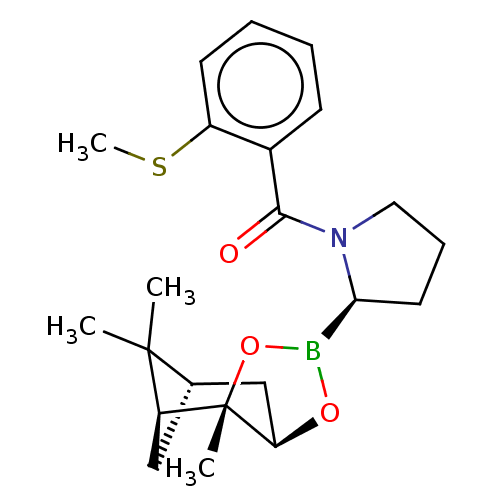 (CHEMBL4576969) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged POP (2 to 710 residues) expressed in SF21 cells using Z-Gly-Pro-AMC as substrate incubated for ... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111783 BindingDB Entry DOI: 10.7270/Q2CV4N01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50180664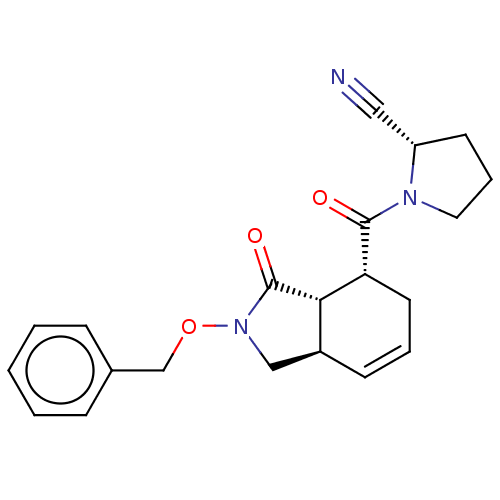 (CHEMBL3818013) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50200733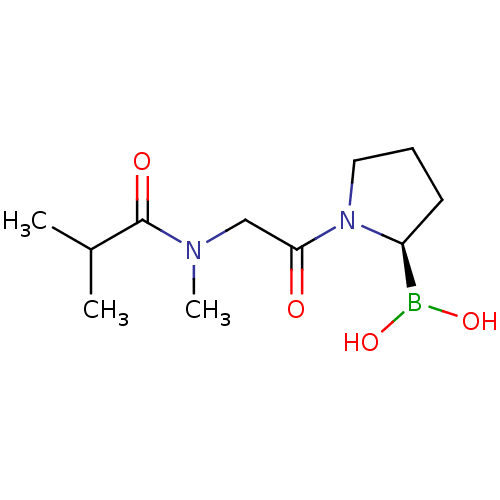 ((R)-1-(2-(N-methylisobutyramido)acetyl)pyrrolidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibitory constant against POP | Bioorg Med Chem Lett 17: 1438-42 (2007) Article DOI: 10.1016/j.bmcl.2006.11.072 BindingDB Entry DOI: 10.7270/Q2VH5NH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human; Moderately active | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human prolyl endopeptidase | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135630 ((S)-2-[(S)-2-(5-Phenyl-isoxazole-3-carbonyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135635 (2-[2-(Isothiazole-3-carbonyl)-pyrrolidine-1-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50374289 (CHEMBL269822) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human prolyl endopeptidase | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135634 ((S)-2-[(S)-2-(5-Trimethylsilanyl-isoxazole-3-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (PO) in human | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50508977 (CHEMBL4451993) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of recombinant human POP assessed as affinity constant of first step of the binding event pre-incubated for 2 hrs before addition of ZGP-p... | J Med Chem 62: 7874-7884 (2019) Article DOI: 10.1021/acs.jmedchem.9b00642 BindingDB Entry DOI: 10.7270/Q2FB5682 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50508979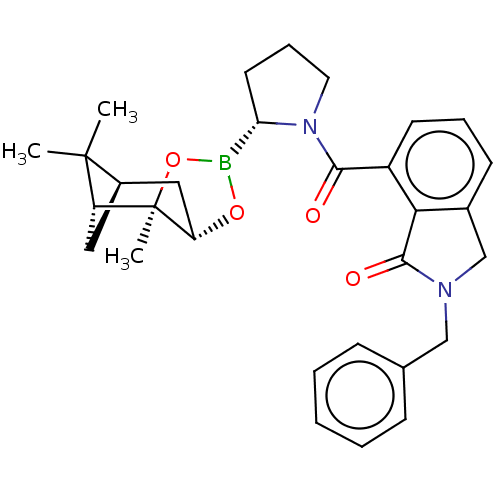 (CHEMBL4558566) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of recombinant human POP pre-incubated for 2 hrs before addition of ZGP-pNA substrate by absorbance assay | J Med Chem 62: 7874-7884 (2019) Article DOI: 10.1021/acs.jmedchem.9b00642 BindingDB Entry DOI: 10.7270/Q2FB5682 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50393843 (CHEMBL2159748) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human POP expressed in Escherichia coli BL21 pre-incubated for 30 mins before ZGP-pNA substrate addition | J Med Chem 59: 4221-34 (2016) Article DOI: 10.1021/acs.jmedchem.5b01296 BindingDB Entry DOI: 10.7270/Q2Z89FBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 114 total ) | Next | Last >> |