

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50211740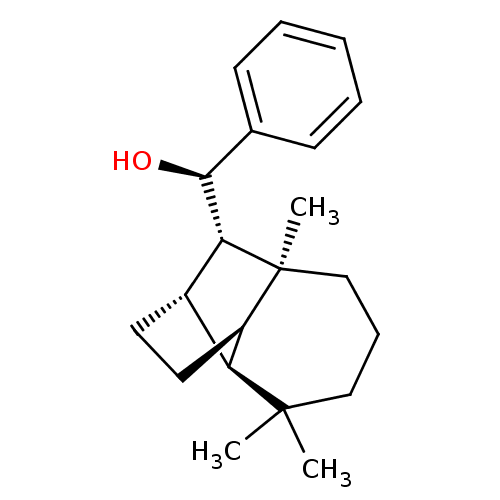 ((1R)-phenyl-[(1R,2S,7S,8S,9S)-3,3,7-trimethyltricy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Inhibition of human UGT2B7 assessed as reduction of estriol glucuronidation | J Med Chem 50: 2655-64 (2007) Article DOI: 10.1021/jm061204e BindingDB Entry DOI: 10.7270/Q23F4QF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50071058 ((2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM19264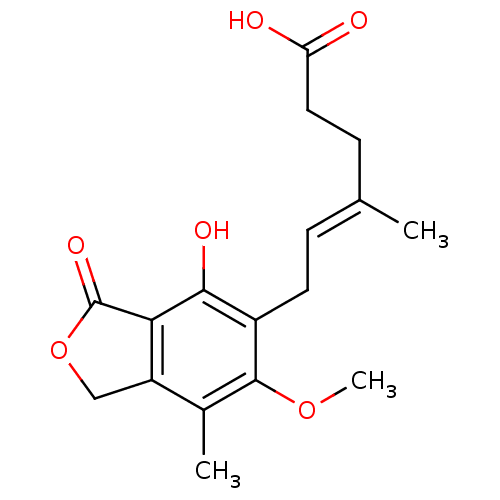 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Uncompetitive substrate inhibition of UGT2B7 in non-diabetic human liver microsomes assessed as reduction in enzyme-mediated mycophenolic acid phenol... | Drug Metab Dispos 39: 448-55 (2011) Article DOI: 10.1124/dmd.110.036608 BindingDB Entry DOI: 10.7270/Q2S46TP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Uncompetitive substrate inhibition of UGT2B7 in diabetic human liver microsomes assessed as reduction in enzyme-mediated mycophenolic acid phenolic 7... | Drug Metab Dispos 39: 448-55 (2011) Article DOI: 10.1124/dmd.110.036608 BindingDB Entry DOI: 10.7270/Q2S46TP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Uncompetitive substrate inhibition of UGT2B7 in diabetic human kidney microsomes assessed as reduction in enzyme-mediated mycophenolic acid phenolic ... | Drug Metab Dispos 39: 448-55 (2011) Article DOI: 10.1124/dmd.110.036608 BindingDB Entry DOI: 10.7270/Q2S46TP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Uncompetitive substrate inhibition of UGT2B7 in non-diabetic human kidney microsomes assessed as reduction in enzyme-mediated mycophenolic acid pheno... | Drug Metab Dispos 39: 448-55 (2011) Article DOI: 10.1124/dmd.110.036608 BindingDB Entry DOI: 10.7270/Q2S46TP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Uncompetitive substrate inhibition of UGT2B7 in diabetic human liver microsomes assessed as reduction in enzyme-mediated mycophenolic acid acyl glucu... | Drug Metab Dispos 39: 448-55 (2011) Article DOI: 10.1124/dmd.110.036608 BindingDB Entry DOI: 10.7270/Q2S46TP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Uncompetitive substrate inhibition of UGT2B7 in diabetic human kidney microsomes assessed as reduction in enzyme-mediated mycophenolic acid acyl gluc... | Drug Metab Dispos 39: 448-55 (2011) Article DOI: 10.1124/dmd.110.036608 BindingDB Entry DOI: 10.7270/Q2S46TP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Uncompetitive substrate inhibition of UGT2B7 in non-diabetic human liver microsomes assessed as reduction in enzyme-mediated mycophenolic acid acyl g... | Drug Metab Dispos 39: 448-55 (2011) Article DOI: 10.1124/dmd.110.036608 BindingDB Entry DOI: 10.7270/Q2S46TP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Uncompetitive substrate inhibition of UGT2B7 in non-diabetic human kidney microsomes assessed as reduction in enzyme-mediated mycophenolic acid acyl ... | Drug Metab Dispos 39: 448-55 (2011) Article DOI: 10.1124/dmd.110.036608 BindingDB Entry DOI: 10.7270/Q2S46TP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50183611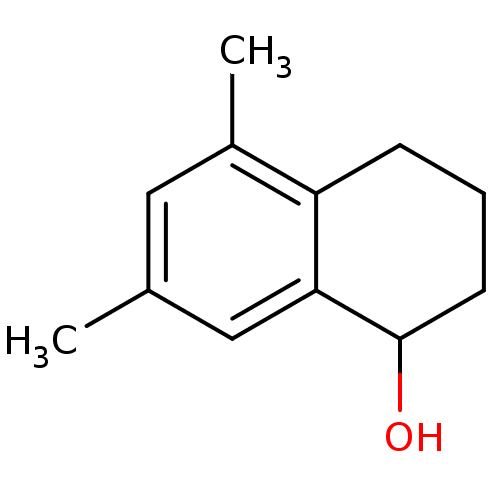 ((rac)-5,7-dimethyl-1,2,3,4-tetrahydronaphthalen-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidation | J Med Chem 49: 1818-27 (2006) Article DOI: 10.1021/jm051142c BindingDB Entry DOI: 10.7270/Q2B857QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50183598 ((rac)-6-methyl-3,4-dihydro-2H-chromen-4-ol | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidation | J Med Chem 49: 1818-27 (2006) Article DOI: 10.1021/jm051142c BindingDB Entry DOI: 10.7270/Q2B857QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50002692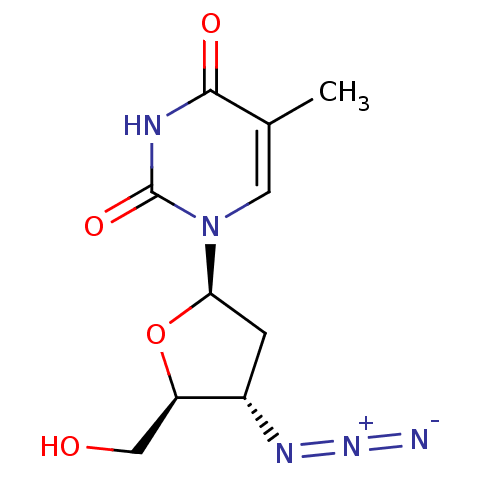 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Uncompetitive substrate inhibition of UGT2B7 in non-diabetic human kidney microsomes assessed as reduction in enzyme-mediated zidovudine glucuronide ... | Drug Metab Dispos 39: 448-55 (2011) Article DOI: 10.1124/dmd.110.036608 BindingDB Entry DOI: 10.7270/Q2S46TP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Uncompetitive substrate inhibition of UGT2B7 in diabetic human kidney microsomes assessed as reduction in enzyme-mediated zidovudine glucuronide form... | Drug Metab Dispos 39: 448-55 (2011) Article DOI: 10.1124/dmd.110.036608 BindingDB Entry DOI: 10.7270/Q2S46TP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50088511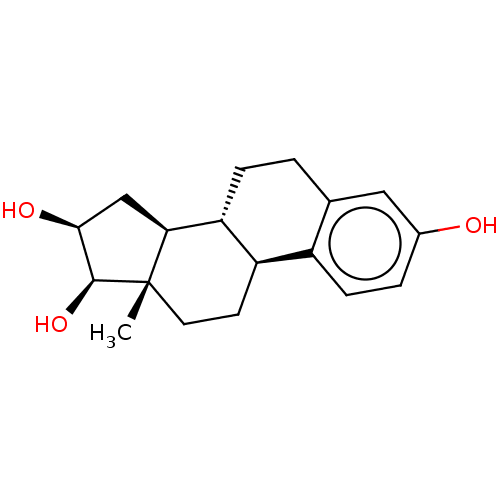 (CHEBI:87620 | Epiestriol | Epioestriol) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged UGT2B7 after 15 to 60 mins by Michaelis-Menten equation analysis in presence of UDPGA | Drug Metab Dispos 41: 582-91 (2013) Article DOI: 10.1124/dmd.112.049072 BindingDB Entry DOI: 10.7270/Q2959K9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50183611 ((rac)-5,7-dimethyl-1,2,3,4-tetrahydronaphthalen-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Non competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidation | J Med Chem 49: 1818-27 (2006) Article DOI: 10.1021/jm051142c BindingDB Entry DOI: 10.7270/Q2B857QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Uncompetitive substrate inhibition of UGT2B7 in non-diabetic human liver microsomes assessed as reduction in enzyme-mediated zidovudine glucuronide f... | Drug Metab Dispos 39: 448-55 (2011) Article DOI: 10.1124/dmd.110.036608 BindingDB Entry DOI: 10.7270/Q2S46TP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island Curated by ChEMBL | Assay Description Uncompetitive substrate inhibition of UGT2B7 in diabetic human liver microsomes assessed as reduction in enzyme-mediated zidovudine glucuronide forma... | Drug Metab Dispos 39: 448-55 (2011) Article DOI: 10.1124/dmd.110.036608 BindingDB Entry DOI: 10.7270/Q2S46TP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50183598 ((rac)-6-methyl-3,4-dihydro-2H-chromen-4-ol | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Non competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidation | J Med Chem 49: 1818-27 (2006) Article DOI: 10.1021/jm051142c BindingDB Entry DOI: 10.7270/Q2B857QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50183609 ((rac)-6-methyl-2,3-dihydro-1H-inden-1-ol | CHEMBL2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidation | J Med Chem 49: 1818-27 (2006) Article DOI: 10.1021/jm051142c BindingDB Entry DOI: 10.7270/Q2B857QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Drug metabolism assessed as recombinant human UGT2B7 assessed as O-glucuronidation measured as inhibition constant at 10 to 400 uM incubated for 60 m... | Drug Metab Dispos 40: 240-8 (2012) Article DOI: 10.1124/dmd.111.042150 BindingDB Entry DOI: 10.7270/Q2KS6T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50183609 ((rac)-6-methyl-2,3-dihydro-1H-inden-1-ol | CHEMBL2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Non competitive inhibition of UGT2B7-catalyzed scopoletin glucuronidation | J Med Chem 49: 1818-27 (2006) Article DOI: 10.1021/jm051142c BindingDB Entry DOI: 10.7270/Q2B857QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 2B7 (Homo sapiens (Human)) | BDBM50003616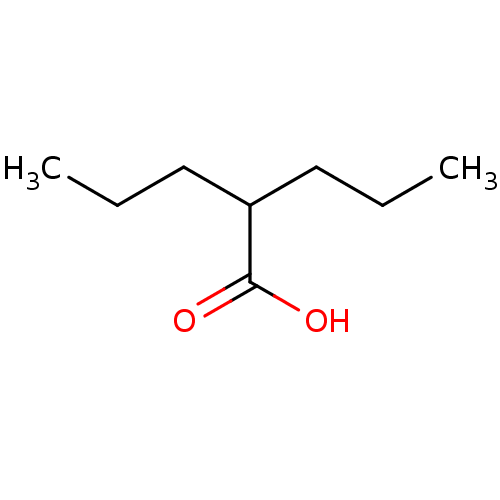 (2-n-propyl-n-valeric acid | CHEMBL109 | DPA | Di-n...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of British Columbia Curated by ChEMBL | Assay Description Inhibition of AZT glucuronidation by human recombinant UGT2B7 | Pharmacol Ther 106: 97-132 (2005) Article DOI: 10.1016/j.pharmthera.2004.10.013 BindingDB Entry DOI: 10.7270/Q2959HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||